Alcohols, Phenols and Ethers
Maharashtra Board-Class-12-Chemistry-Chapter-11
Notes-Part-1
|
Topics to be Learn : Part-1
|
Introduction :
Alcohols are organic compounds whose molecules contain hydroxyl group, (-OH) attached to a saturated carbon atom.
- Examples : CH3—OH methyl alcohol, CH3—CH2-OH ethyl alcohol.
Classification of alcohol :
Depending on the basis of hydroxyl groups present in a molecule, alcohols are classified into monohydric, dihydric, trihydric and polyhydric alcohols.
Monohydric alcohols : Alcohols having only one hydroxyl group in their molecules are called monohydric alcohols. e.g., CH3-OH
- Monohydric alcohol are classified according to the type of hybridization of the carbon atom to which the hydroxyl group is attached.
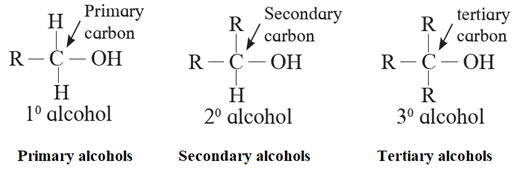
(i) Alcohols containing sp3C—OH bond : In these alcohols —OH group is attached to a sp3-hybridised carbon atom of alkyl group.
- These alcohols are represented as R—OH. They are further classified as primary, secondary and tertiary alcohols in which —OH group is attached to primary, secondary and tertiary carbon atoms respectively.
- Each of these three types of alcohols can also be either allylic or benzylic if the sp3 carbon carrying -OH is further bonded to sp2
(a) Allylic alcohols : In these alcohols —OH group is attached to a sp3 -hybridised carbon atom next to the carbon-carbon double bond i.e., to allylic carbon. Allylic alcohols may be primary, secondary and tertiary alcohols.
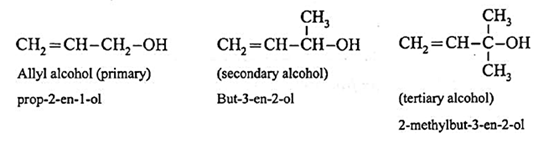
(b) Benzylic alcohols : In these alcohols —OH group is attached to a sp3-hybridised carbon atom next to an aromatic ring. Benzylic alcohols may be primary, secondary and tertiary alcohols.

(ii) Alcohols containing sp2C—OH bond : In these alcohols —OH group is attached to a sp2-hybridised carbon atom, i.e., vinylic carbon. These alcohols are also called vinylic alcohols. e.g., CH2=CH-OH vinyl alcohol.
Phenols (carbolic acids or benzenol) :
Hydroxy derivatives of aromatic hydrocarbons in which the hydroxyl group is directly attached to the aromatic ring are called phenols.
Phenols are classified on the basis of number of hydroxyl (—OH) groups present in a molecule of phenol.
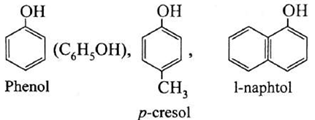
(i) Monohydric phenols : Phenols contain one hydroxyl group in their molecule.
Examples :
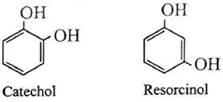
(ii) Dihydric phenols : Phenols contain two hydroxyl groups in their molecule.
Examples :
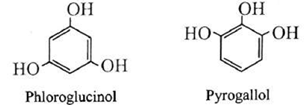
(iii)Trihydric phenols : Phenols contain three hydroxyl groups in their molecule.
Examples :
Question : Classifiy the following alcohols as 10 / 20 / 30 and allylic/benzylic
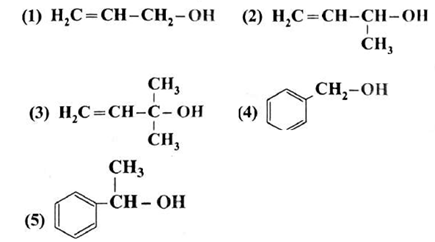
(1) Allylic alcohol (primary) (2) Allylic alcohol (secondary) (3) Allylic alcohol (tertiary) (4) Benzylic alcohol (primary) (5) Benzylic alcohol (secondary)
Ethers :
They are alkoxy derivatives of alkanes in which a hydrogen atom of alkane (R—H) is replaced by alkoxy group (—O—R) or divalent oxygen atom is attached to two alkyl groups or two aryl groups or one alkyl and one aryl group.
- Ethers are organic oxides R—O—Ar, Ar—O—Ar. E.g. R—O—R or C2H5—O—C2H5
- The general formula of ethers is CnH2n+2 For example, dimethyl ether CH3—O—CH3 has molecular formula C2H6O.
Classification of Ethers : Ethers are classified into two groups as symmetrical ethers (simple ethers) or unsymmetrical ethers (mixed ethers) depending on whether the two alkyl/aryl groups bonded to oxygen atom are same or different respectively.
(i) Simple or symmetrical ether : The ethers in which both alkyl (or aryl) groups attached to the oxygen atom are same are called simple ethers.
- g. (R-O-R), CH3—O—CH3, dimethyl ether; C6H5—O—C6H5; diphenyl ether.
(ii) Mixed or unsymmetrical ethers : The ethers in which the two alkyl (or aryl) groups attached to the oxygen atom are different are called mixed ethers.
- g. (R-O-R’), CH3—O—C2H5, ethyl methyl ether; C2H5— O—C6H5 ethyl phenyl ether.
Nomenclature :
Common nomenclature system :
- In this system, monohydric alcohols (R—OH) are named as alkyl alcohols.
- According to the attachment of hydroxyl group to a carbon atom they are named with prefixes as n-(normal or primary) alcohol, sec-(secondary) alcohol, tert-(tertiary) alcohol.
- Alcohols with two hydroxyl groups are named as glycols.
Example :

Carbinol system : In this system alcohols are considered as derivatives of methyl alcohol which is called Carbinol. The alkyl group attached to the carbon carrying —OH group are named in alphabetical order. Then the prefix carbinol is added.
IUPAC system of nomenclature :
- In this system, alcohols are named as alkanols.
- A longest continuous chain of carbon atoms containing —OH group is chosen as a parent hydrocarbon and alcohol is considered as a hydroxy derivative of this alkane.
- The carbon atoms are numbered from a terminal carbon atom nearest to a carbon atom attached to —OH group so that the position of —OH group is indicated by the lowest locant.
- e’ of an alkane is preplaced by ‘ol’, giving alkanol. The number of OH groups is indicated by prefix, di, tri, etc. before ‘ol’. The positions of —OH groups are indicated by appropriate locants.
- The different substituents are arranged in the alphabetical order, and their positions are indicated by proper numbers.
- Their names are hyphened on either sides except the last substituent.
- For cyclic alcohols are named by using prefix cyclo to the parent alkane considering —OH group attached to carbon atom C—1.
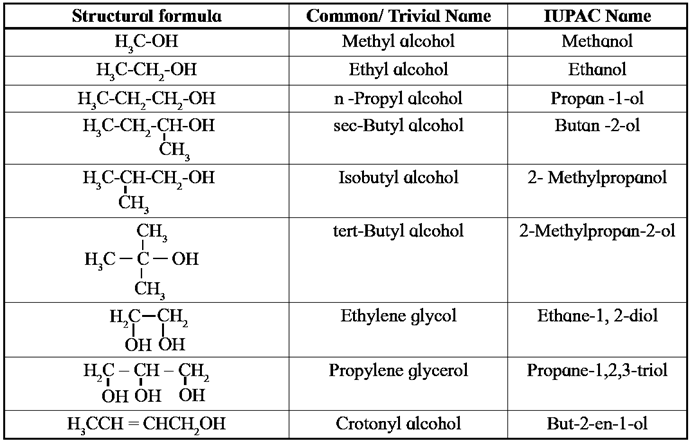
Common/Trivial and IUPAC Names of some alcohols
Nomenclature of Phenols :
- The IUPAC system name of phenol is benzenol. The common name phenol is also accepted by IUPAC.
- The common names have prefixes ortho, meta and para in substituted phenols. IUPAC system uses the locant 2-, 3-, 4-, etc. to indicate the positions of substituents.
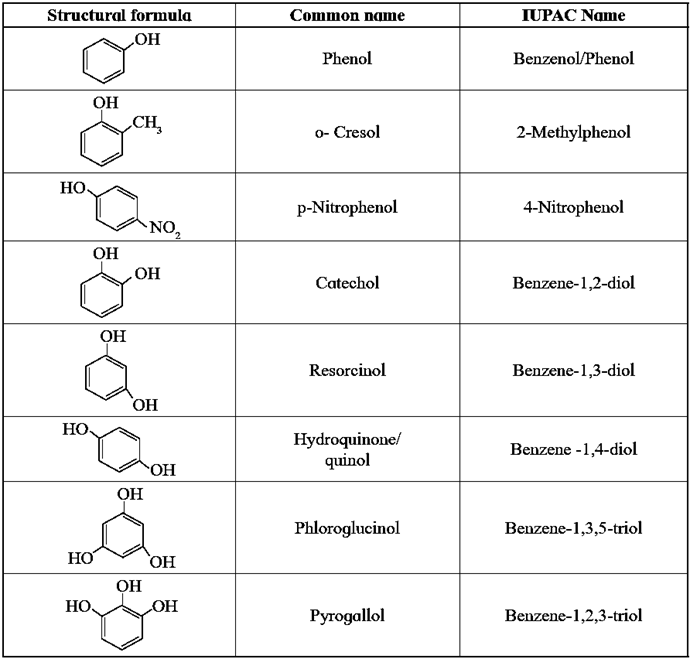
Common and IUPAC names of some phenols :
Nomenclature of Ethers :
Common and IUPAC system of nomenclature of ethers :
- The term ether is added to the names of the alkyl groups connected to the oxygen atom in the conventional system of naming to designate ethers. Prefix di- is used if two alkyl groups are identical.
- Ethers are known as alkoxyalkanes in the nomenclature used by the IUPAC. The parent alkane is considered to be the bigger alkyl group. The name of the alkoxy group and its locant are affixed to the name of the smaller alkane.
Common and IUPAC Names of some Ethers :

Question : Classify the following alcohols as primary, secondary and tertiary and write their IUPAC names.
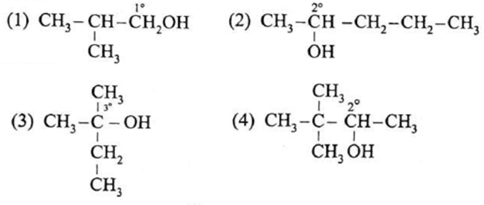
(1) Primary alcohol – IUPAC Name : 2-Methylpropan-1-ol (2) Secondary alcohol – IUPAC Name : Pentan-2-ol (3) Tertiary alcohol – IUPAC Name : 2-Methylbutan-2-ol (4) Secondary alcohol – IUPAC Name : 3, 3-Dimethylbutan-2-ol
Question : Write IUPAC names of the following compounds.
(i) Cyclobutane-1, 1-diol (ii) 2-Ethoxybutane (iii) 2-Phenylethan-1-ol (iv) 3-Methylphenol (m-cresol)
Preparation of Alcohols and Phenols :
Preparation of alcohols :
(1) From alkyl halide by hydrolysis with aqueous alkali or moist silver oxide
(i) Alkyl halide converted into alcohol by using Aqueous NaOH (or KOH) :
When an alkyl halide (R—X), is boiled with aqueous NaOH (or KOH) an alcohol is obtained,
R—X + NaOH (aq) \(\underrightarrow{boil}\) R—OH + NaX
[R—X + KOH (aq) \(\underrightarrow{boil}\) R—OH + KX]
(ii) Alkyl halide when heated with moist Ag2O, undergoes hydrolysis and forms an alcohol.
Ag2O + H2O → 2AgOH
R—X + AgOH \(\underrightarrow{Δ}\) R—OH + AgX
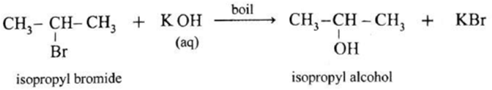
Ethanol : When ethyl bromide (bromoethane) is refluxed with aqueous potassium hydroxide, ethyl alcohol is formed. The reaction is called a hydrolysis reaction. CH3—CH2—Br + KOH \(\underrightarrow{boil}\) CH3—CH2—OH + KBr Isopropyl alcohol : When isopropyl bromide (2-bromopropane) is boiled with aqueous potassium hydroxide, isopropyl alcohol is formed. Butan-2-ol : When 2-Chlorobutane is boiled with aqueous KOH, Butan-2-ol is obtained.
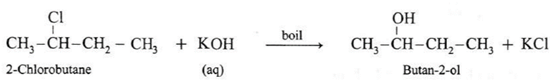
Alkyl halides using moist silver oxide :
(i) Bromoethane (C2H5Br) when boiled with moist Ag,0 undergoes hydrolysis and forms C2H5OH.
Ag2O + H2O → 2AgOH
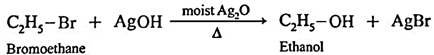
(2) By acid catalyzed hydration of alkenes or olefins :
The addition of a water molecule across the double bond in an alkene is called hydration of alkenes or olefins.

Hydration does not take place directly. Alkene reacts with sulfuric acid to produce alkyl hydrogen sulfate, which on hydrolysis gives alcohol. This reaction follows Markownikoff’s rule.

CH2=CH2 + HOSO3H → CH3-CH2-OSO3H CH3-CH2-OSOH + H2O \(\underrightarrow{\text{ boil }}\) CH3-CH2-OH + HOSO3H

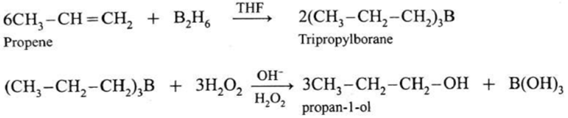
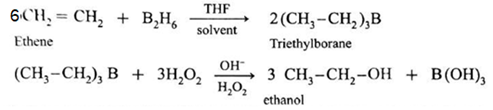
(3) By Hydroboration - Oxidation of alkenes :
When diborane is treated with alkene in the presence of tetrahydrofuran (THF ) solvent, an addition product trialkyl borane is formed. Trialkyl borane is then oxidised with alkaline peroxide forms primary alcohol.
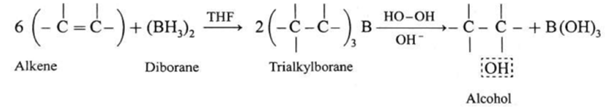
The addition of diborane to the double bond takes place in such a way that the boron gets attached to the less substituted carbon. The overall reaction gives Anti-Markovnikov’s product from unsymmetrical alkenes.
(4) By reduction of carbonyl compounds, by reduction of aldehydes and ketones :
Aldehyde and ketones are carbonyl compounds containing a carbonyl group >C = O.
(i) Aldehydes on reduction by H2/Ni or LiAlH4 give primary alcohols (10).
R—CHO (Aldehyde) + H2(g) \(\underrightarrow{Δ, Ni}\) R—CH2OH (Primary alcohol)
- Example : When acetaldehyde is reduced with hydrogen in the presence of nickel as catalyst and at high temperature, ethyl alcohol (ethanol) is obtained.

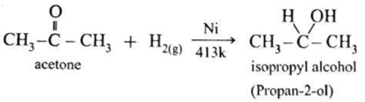
(ii) Similarly, ketones on reduction with H2/Ni or LiAlH4 give secondary alcohols (20).
- Example : When acetone is reduced with hydrogen in the presence of nickel as catalyst and at high temperature isopropyl alcohol (propan-2-ol) is obtained.
(5) By reduction of carboxylic acids :
Carboxylic acids and esters are not easily reduced by catalytic hydrogenation or by NaBH. Caboxylic acids require strong reducing agent LiAlH4 to form primary alcohols.
When acetic acid is reduced in the presence of LiAlH4 and followed by their acid hydrolysis, ethyl alcohol is obtained.

When ethyl acetate is reduced in the presence of LiAlH4 and followed by their acid hydrolysis, n-propyl alcohol and ethyl alcohol is obtained.
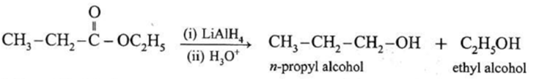
However LiAlH4 is an expensive reagent. Therefore, commercially acids are first transformed into esters which on catalytic hydrogenation give primary alcohols.

Crotonyl alcohol obtained from crotonaldehyde :
When crotonaldehyde is reduced in the presence of lithium aluminium hydride, the product obtained is hydrolysed to give crotonyl alcohol. Here, LiAlH4 does not reduce carbon-carbon double bond.
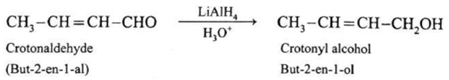
| Know This :
The advantage of LiAlH4 over H2/Ni is that it does not reduce the isolated olefinic bond and hence it can reduce unsaturated aldehyde and ketones to unsaturated alcohols. |
(6) By addition of Grignard reagent to aldeheydes and ketones : Grignard reagent reacts with aldehyde or ketone to form an adduct which on hydrolysis with dilute acid gives the corresponding alcohols.
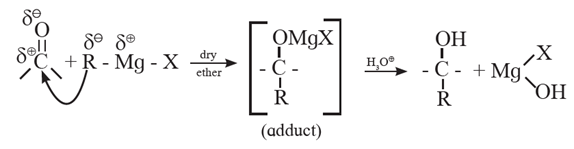
In the first step, the nucleophilic addition of Grigard reagent to the carbonyl group resulting in the formation of an adduct, which on hydrolysis yields an alcohol.
Examples : Ethanol : Formaldehyde on reaction with Grignard reagent, CH3—Mg—I in dry ether forms a complex which on further hydrolysis with dilute HCl forms ethanol. Propanol-1-ol : Formaldehyde on reaction with Grignard reagent, CH3—Mg—I in dry ether forms a complex which on further hydrolysis with dilute HCl forms Propan-1-ol. 2-Methyl propan-2-ol : Acetone on reaction with Grignard reagent in dry ether forms a complex which on further hydrolysis with dilute acid HCI, forms a tertiary butyl alcohol.
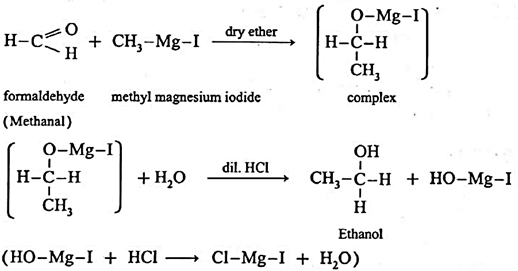
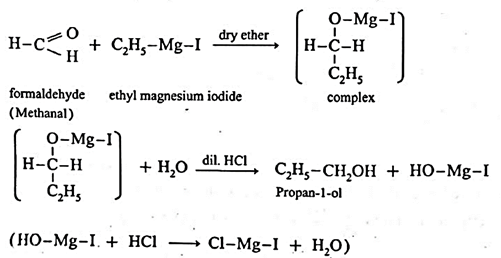
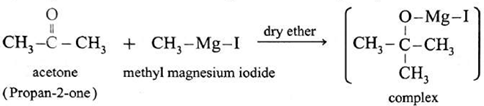

Synthesis of alcohols :
This reaction is useful in synthesis of a variety of alcohols (see Table)
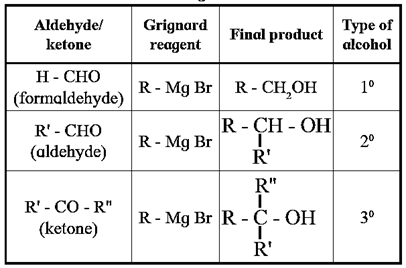
Examples : Using Grignard reagent synthesis of following alcohols from aldehydes or ketones : (b) Synthesis of propan-1-ol :


Preparation of phenol :
(i) Preparation of phenol from chlorobenzene (Dow Process) :
Chlorobenzene is fused with NaOH at high temperature and pressure (623K and 150atm) followed by treatment with dilute HCl to obtain phenol.
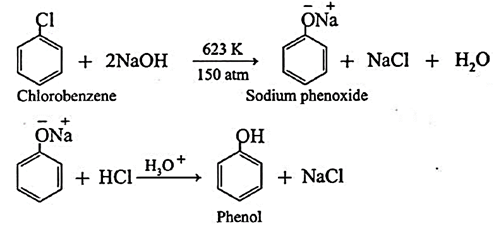
(ii) Preparation of phenol from Cumene :
- This is the commercial method of preparation of phenol.
- When a stream of air is passed through cumene (isopropylbenzene) suspended in aqueous Na2CO3 solution in the presence of cobalt naphthenate catalyst, isopropylbenzene hydroperoxide or cumene hydroperoxide is formed.
- Isopropylbenzene hydroperoxide on warming with dil. HCl gives phenol and acetone.
- Acetone is an important by-product of the reaction and is separated by distillation.
- The reaction is called auto oxidation.

(iii) Preparation of phenol from benzene sulphonic acid :
- Benzene sulphonic acid is neutralized with the requisite quantity of soda ash (Na2CO3) or NaOH and the solution is evaporated to obtain sodium benzene sulphonate salt.
- Dry sodium benzene sulphonate is fused with an excess of caustic soda (NaOH) at about 573 K when sodium phenoxide is formed.
- The fused mass of sodium phenoxide on treatment with dilute HCl gives phenol.

(iv) Preparation of phenol from aniline (diazotization) :
- When aniline is treated with sodium nitrite and hydrochloric acid (NaNO2+HCl) at low temperature (0°C—5°C), benzene diazonium chloride is formed.
- This reaction is called diazotization. An aqueous solution of benzene diazonium chloride on warming with water or dil. H2SO4 gives phenol.

PDF : Chapter-11-Alcohols, Phenols and Ethers-Text Book
PDF : Chapter-11-Alcohols, Phenols and Ethers- Notes
PDF : Chapter-11-Alcohols, Phenols and Ethers- Solution
All 16 Chapters Notes -Class-12-Chemistry (16-PDF)
All 16 Chapters Solutions -Class-12-Chemistry (16-PDF)
All 16 Chapters Notes+Solutions -Class-12-Chemistry (32-PDF)
Main Page : – Maharashtra Board Class 12th-Chemistry – All chapters notes, solutions, videos, test, pdf.
Previous Chapter : Chapter 10- Halogen Derivatives – Online Notes
Next Chapter : Chapter-12-Aldehydes, Ketones and Carboxylic acids- Online Notes
We reply to valid query.