Redox Reactions
Maharashtra State Board-Class-11-Science-Chemistry-Chapter -6
Solutions
Question 1. Choose the most correct option
(A) Oxidation numbers of Cl atoms marked as Cla and Clb in CaOCl2 (bleaching Powder) are
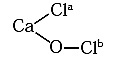
(a) zero in each
(b) -1 in Cla and +1 in Clb
(c) +1 in Cla and -1 in Clb
(d) 1 in each
(b) -1 in Cla and +1 in Clb
(B) Which of the following is not an example of redox reaction?
(a) CuO + H2 → Cu + H2O
(b) Fe2O3 + 3CO2 → 2Fe + 3CO2
(c) 2K + F2 → 2KF
(d) BaCl2 + H2SO4 → BaSO4 + 2HCl
(d) BaCl2 + H2SO4 → BaSO4 + 2HCl
(C) A compound contains atoms of three elements A, B and C. If the oxidation State of A is +2, B is +5 and that of C is -2, the compound is possibly represented by
(a) A2 (BC3)2
(b) A3 (BC4)2
(c) A3 (B4C)2
(d) ABC2
(b) A3 (BC4)2
(D) The coefficients p, q, r, s in the reaction
p Cr2O72+ + q Fe2+ → r Cr3+ + s Fe3+ + H2O respectively are :
(a) 1, 2, 6, 4
(b) 6, 1, 2, 4
(c) 1, 6, 2, 6
(d) 1, 2, 4, 6
(c) 1, 6, 2, 6
(E) For the following redox reactions, find the correct statement.
Sn2+ + 2Fe3+ Sn4+ + 2Fe2+
(a) Sn2+ is undergoing oxidation
(b) Fe3+ is undergoing oxidation
(c) It is not a redox reaction
(d) Both Sn2+ and Fe3+ are oxidized
(a) Sn2+ is undergoing oxidation
(F) Oxidation number of carbon in H2CO3 is
(a) +1
(b) +2
(c) +3
(d) +4
(d) +4
(G) Which is the correct stock notation for manganese dioxide?
(a) Mn(I)O2
(b) Mn(II)O2
(c) Mn(III)O2
(d) Mn(IV)O2
(d) Mn(IV)O2
(I) Oxidation number of oxygen in superoxide is
(a) -2
(b) -1
(c) \(-\frac{1}{2}\)
(d) 0
(c) \(-\frac{1}{2}\)
(J) Which of the following halogens does always show oxidation state -1?
(a) F
(b) Cl
(c) Br
(d) I
(a) F
(K) The process SO2 → S2Cl2 is
(a) Reduction
(b) Oxidation
(c) Neither oxidation nor reduction
(d) Oxidation and reduction.
(a) Reduction
(2) Write the formula for the following Compounds:
(A) Mercury(II) chloride
(B) Thallium(I) sulphate
(C) Tin(IV) oxide
(D) Chromium(III) oxide
| Compound | Formula |
| Mercury(II) chloride | HgCl2 |
| Thallium(I) sulphate | Tl2SO4 |
| Tin(IV) oxide | SnO2 |
| Chromium(III) oxide | Cr2O3 |
(3) Answer the following questions
(A) In which chemical reaction does carbon exhibit variation of oxidation state from -4 to +4 ? Write balanced chemical reaction.
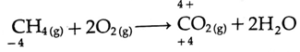
(B) In which reaction does nitrogen exhibit variation of oxidation state from -3 to +5 ?
![]()
(C) Calculate the oxidation number of underlined atoms.
(a) H2SO4
H2SO4 is a neutral molecule.
Sum of oxidation number of all atoms of H2SO4 = 0
∴ 2(oxidation number of H) + oxidation number of S + 4(oxidation number of O) = 0
∴ 2(+1) + x + 4(-2) = 0
∴ x = + 6
∴ Oxidation number of S = +6
(b) HNO3
HNO3 is a neutral molecule.
Sum of oxidation number of all atoms of HNO3 = 0
∴ Oxidation number of H + oxidation number of N +3(oxidation number of O) = 0
∴ (+1) + x + 3(—2) = 0
∴ x = + 5
∴ Oxidation number of N = +5
(c) H3PO3
H3P03 is a neutral molecule.
Sum of oxidation number of all atoms of H3PO3 = 0
∴ 3(oxidation number of H) + oxidation number of P + 3(oxidation number of O) = 0
∴ 3(+1) + x + 3(—2)
∴ x = + 3
∴ Oxidation number of P = + 3
(d) K2C2O4
K2C2O4 is a neutral molecule.
Sum of oxidation number of all atoms of K2C2O4 = 0
∴ 2(oxidation number of K) +2(oxidation number of C) + 4(oxidation number of O) = 0
∴ 2(+1) + 2x + 4(—2) = 0
∴ x = + 3
∴ Oxidation number of C = + 3
(e) H2S4O6
H2S4O6 is a neutral molecule.
Sum of oxidation number of all atoms of O H2S4O6 = 0
∴ 2(oxidation number of H) + 4(oxidation number of S) + 6(oxidation number of O) = 0
∴ 2(+1) + 4(x) + 6(—2) = 0
∴ x = + 2.5
∴ Oxidation number of S = + 2.5
(f) Cr2O72—
Cr2O72— is an ionic species carrying charge —2
Sum of oxidation number of all atoms 0f Cr2O72— = —2
∴ 2(oxidation number of Cr) + 7(oxidation number of O) = — 2
∴ 2x + 7(—2) = -2
∴ x = +6
∴ Oxidation number of Cr = + 6
(g) NaH2PO4
NaH2PO4 is a neutral molecule.
Sum of oxidation number of all atoms of NaH2PO4 = 0
∴ Oxidation number of Na + 2(oxidation number of H) + oxidation number of P + 4(oxidation number of O) = 0
∴ (+1) + 2(+1) + x + 4(—2) = 0
∴ x = + 5
∴ Oxidation number of P = +5
(D) Justify that the following reactions are redox reaction; identify the species oxidized/reduced, which acts as an oxidant and which act as a reductant.
(a) 2Cu2O (s) + Cu2S (s) → 6Cu (s) + SO2 (g)

(i) The reaction is a redox reaction
(ii) Cu is reduced from + 1 to zero
(iii) S is oxidized from — 2 to + 4
(iv) Cu2O is an oxidant
(v) Cu2S is a reductant
(b) HF (aq) + OH— (aq) → H2O (l) + F— (aq)
Since there is no change in the oxidation number of any element it is not a redox reaction.
![]()
(c) I2 (aq) + 2S2O32— (aq) → S4O62— (aq) + 2I— (aq)
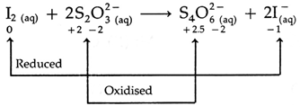
(i) The reaction is a redox reaction
(ii) I is reduced from zero to — 1.
(iii) S is oxidized from + 2 to + 2.5
(iv) I2 is an oxidant.
(v) S2O32- Is a reductant.
(E) What is oxidation? Which one of the following pairs of species is in its oxidized state?
(a) Mg / Mg2+
(b) Cu / Cu2+
(c) O2 / O2
(d) Cl2 / Cl
Oxidation is defined as:
- Addition of oxygen.
- Addition of electronegative element.
- Removal of hydrogen.
- Removed of electropositive element.
- Loss of electrons by any species.
The species in the oxidized state
(a) Mg2+ (b) Cu2+ (c) O2 (d) Cl2
(F) Justify the following reaction as redox reaction.
2Na(s) + S(s) → Na2 S(s)
Find out the oxidizing and reducing agents.
![]()
(i) The reaction is a redox reaction.
(ii) S(S) is an oxidizing agent.
(iii) Na(S) is a reducing agent.
(iv) Na(S) is oxidizing and S(S) is reduced
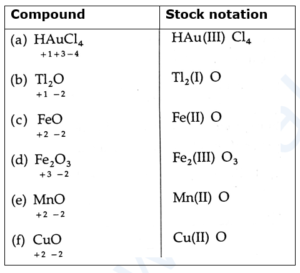
(G) Provide the stock notation for the following compounds: HAuCl4, Tl2O, FeO, Fe2O3, MnO and CuO.
(H) Assign oxidation number to each atom in the following species.
(a) Cr(OH)4
Cr(OH)4 is an ionic species carrying net charge —1.
Sum of oxidation number of all atoms of Cr(OH)4 = —1
∴ x + 4(oxidation number of O) + 4(oxidation number of H) = — 1
∴ x + 4(—2) + 4(+1) = —1
∴ x = + 3
∴ Oxidation number of Cr = + 3
(b) Na2S2O3
Na2S2O3 is a neutral molecule.
Sum of oxidation number of all atoms of Na2S2O3 = 0
∴ 2(oxidation number of Na) + 2(oxidation number of S) + 3(oxidation number of O) = 0
∴ 2(+1) + 2(x) + 3(—2) = 0
∴ x = + 2
∴ Oxidation numbers of S = + 2
(c) H3BO3
H3BO3, is a neutral molecule.
Sum of oxidation number of all atoms of H3BO3 = 0
∴ 3(oxidation number of H) + (oxidation number of B) + 3(oxidation number of O) = 0
∴ 3(+1) + x + 3(-2) = 0
∴ x = + 3
∴ Oxidation number of B = +3.
(I) Which of the following redox couple is stronger oxidizing agent?
(a) Cl2 (E0 = 1.36 V) and Br2 (E0 = 1.09 V)
(b) MnO4— (E0 = 1.51 V) and Cr2O72— (E0 = 1.33 V)
(a) Since the standard reduction potential, E° of C12 (+ 1.36 V) is higher than E° of Br2 (1.09 V) the stronger oxidizing agent in the redox couple is Cl2.
(b) Since standard reduction potential E° for MnO4— (1.51 v) is higher than 15° for Cr2O72— (1.33 V), MnO4— will be a stronger oxidizing agent than Cr2O72—, in the redox couple.
(J) Which of the following redox couple is stronger reducing agent?
(a) Li (E0 = - 3.05 V) and Mg (E0 = - 2.36 V)
(b) Zn (E0 = - 0.76 V) and Fe (E0 = - 0.44 V)
(a) Since the standard reduction potential, E° of Li(— 3.05 V) is less or more negative than E° of Mg (— 2.36 V) lithium will be a stronger reducing agent than. Mg in the redox couple.
(b) Since the standard reduction potential of Zn(— 0.76 V) is less or more negative than E° of Fe (— 0.44 V) zinc will be a stronger reducing agent than Fe in the redox couple.
(4) Balance the reactions/equations:
(A) Balance the following reactions by oxidation number method
(a) Cr2O72—(aq) + SO3 2(aq) → Cr3+(aq) + SO42(aq) (acidic)
(Note : Cr2O22— is given in text book is changed to Cr2O72—)
Step 1: Assign oxidation number to each atom in the given reaction.

Step 2: Identify the atoms that have changed oxidation numbers.
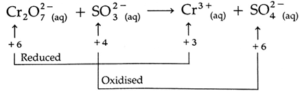
Step 3: Find the total decrease in oxidation number of the reduced atom Cr and total increase in oxidation number of the oxidized atom S.
Total decrease in oxidation number = (+6) — (+3) = 3
Total increase in oxidation number = (+6) — (+4) = 2
Step 4: To balance the net change in oxidation numbers, multiply Cr2O72- by 1 and Cr3+ by 2 and multiply SO32- and SO42- by 3.
Cr2O72-(aq) + 3SO32-(aq) → 2Cr3+(aq) + 3SO42-(aq)
Step 5: Find net charges on left hand and right hand side of the equation.
Total change on left hand side
= (—2) + 3(-2)= — 8
Total change on right hand side
= 2(+3) + 3(—2) = 0
Since there is net — 8 change on left hand side, add 8H+ to it since the reaction is in acidic medium.
Hence balanced equation is,
![]()
(b) MnO4—(aq) + Br—(aq) → MnO2(s) + BrO3—(aq) (basic)
Step 1: Assign oxidation number to each atom in the equation.

Step 2: Identify the atoms that have changed oxidation numbers.

Step 3: Find the total decrease in oxidation number of reduced atom Mn and total increase in oxidation number of oxidized atom Br.
Total decrease in oxidation number
= (+7) — (+4) = 3
Total increase in oxidation number
= (+5) — (—1) = 6
Step 4: To balance the net change in oxidation numbers, multiply MnO4— and MnO2 by 2.
2MnO4—(aq) + Br—(aq) → 2MnO2(aq) + BrO3—(aq)
Step 5: Since there are 3 negative charges on left hand side and 1 negative charge on right hand side of the equation add 1H2O on left and 2OH— on right hand side.
This is a balanced equation.
2MnO4—(aq) + Br—(aq) + H2O(l) → 2MnO2(aq) + BrO3—(aq) + 2OH—(aq)
(c) H2SO4 (aq) + C (s) → CO2 (g) + SO2 (g) + H2O (l) (acidic)
Step 1: Assign oxidation number to each atom in the given equation.
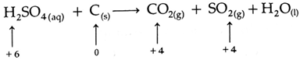
Step 2: Identify the atoms that have changed oxidation numbers.

Step 3: Find the total decrease in oxidation number of reduced atom S and total increases in oxidation number of the oxidized atom C.
Total decrease in oxidation number
= (+6) — (+4) = 2
Total increase in oxidation number
= (+ 4) — (9) = 4
Step 4: To balance the net charge in oxidation numbers, multiply H2SO4 and SO2 by 2.
2H2SO4 (aq) + C (s) → CO2 (g) + 2SO2 (g) + H2O (l)
Since there are 4H on left side and 2H on right side, multiply H2O by 2.
∴ 2H2SO4 (aq) + C (s) → CO2 (g) + 2SO2 (g) + 2H2O (l)
Step 5: Since there is no net charge on left and right hand sides, the above equation is balanced.
(d) Bi(OH)3 (g) + Sn(OH)3— (aq) → Bi (s) + Sn(OH)62— (aq) (basic)
Step 1: Assign oxidation number to each atom in the equation.

Step 2: Identify the atoms that have changed oxidation number.
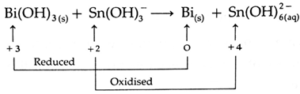
Step 3: Find the total decrease in oxidation number of reduced atom Bi and total increase in oxidation number of oxidized atom Sn.
Total decrease in oxidation number
= (+ 3) — (0) = 3
Total increase in oxidation number
= (+4) — (+2) = 2
Step 4: To balance the net charge in oxidation numbers, multiply Bi(OH)3 and Bi by 2 while Sn(OH)3— and Sn(0H)62— by 3.
2Bi(OH)3 (g) + 3Sn(OH)3 (aq) → 2Bi (s) + 3Sn(OH)62— (aq)
Step 5: Since there are 3 negative charges on left hand side and 6 negative charges on right hand side of the equation, add 3OH- for basic medium on left hand side.
2Bi(OH)3 (g) + 3Sn(OH)3 (aq) + 3OH— (aq) → 2Bi (s) + 3Sn(OH)62— (aq)
This is a balanced equation.
(B) Balance the following redox equation by half reaction method.
(a) H2C2O4 (aq) + MnO4— (aq) → CO2 (g) + Mn2+ (aq) (acidic)
Step 1: Assign oxidation numbers to all atoms

Step 2: Divide the given equation into two half equations, one for oxidation and the other for reduction.
C3+ → CO2 (oxidation half equation)
7+MnO- → Mn2+ (reduction half reaction)
Step 3:
Loss of electrons by C atom = (+ 4) — (+ 3) = 1
Gain of electrons by Mn atom = (+ 7) — (+ 2) = 5
Hence we can write,
H2C2O4 (aq) → 2CO2 + 2e— (oxidation)
MnO4— (aq) + 5e— → Mn2+ (reduction)
Step 4 : T0 balance loss and gain of electrons, multiply oxidation equation by 5 and reduction equation by 2.
5H2C2O4 (aq) → 10CO2 + 10e— (oxidation)
2MnO4— (aq) + 10e— → 2Mn2+ (reduction)
Step 5: By adding both the equations,
5H2C2O4 (aq) + 2MnO4— (aq) → 2Mn2+ + 10CO2 (g)
Step 6: To balance the charges and H atoms add 6H+ on left hand side of equation.
5H2C2O4 (aq) + 2MnO4— (aq) + 6H+ → 2Mn2+ + 8H2O + 10CO2 (g)
The above equation is a balanced equation.
(b) Bi(OH)3 (s) + SnO22— (aq) → SnO32— (aq) + Bi (s) (basic)
Step 1 : Assign oxidation numbers to all atoms.
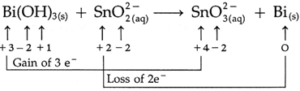
Step 2 : Divide the equation into two half equations one for oxidation and the other for reduction.
SnO22— (aq) → SnO32— (aq) (oxidation half reaction)
Bi(OH)3 (s) → Bi (s) (reduction half reaction)
Step 3 : Loss of electrons by Sn = ( + 4) — (+ 2) = 2
Gain of electrons by Bi = (+ 3) — (O) = 3
Hence we can write,
SnO22— (aq) → SnO32— (aq) + 2e— (Oxidation)
Bi(OH)3 (s) + 3e— → Bi (reduction)
Step 4 : To balance loss and gain of electrons multiply oxidation equation by 3 and reduction equation by 2.
3SnO22— (aq) → 3SnO32— (aq) + 6e— (Oxidation)
2Bi(OH)3 (s) + 6e— → 2Bi (reduction)
Step 5 : By adding both the equations,
3SnO22— (aq) + 2Bi(OH)3 (s) → 3SiO32— (aq) + 2Bi
Step 6 : To balance H and O atoms add 3H2O on right hand side.
3SnO22— (aq) + 2Bi(OH)3 (s) → 3SnO32— (aq) + Bi (s) + 3H2O (l)
(5) Complete the following table:
Assign oxidation number to the underlined species and write Stock notation of compound.
| Compound | Oxidation Number | Stock Notation |
| AuCl3 | ||
| SnCl2 | ||
| V2O74- | ||
| PtCl62- | ||
| H3AsO3 |
| Compound | Oxidation Number | Stock Notation |
| AuCl3 | + 3 | Au(III)Cl3 |
| SnCl2 | + 4 | Sn(II)Cl2 |
| V2O74- | + 5 | V2(V)O74- |
| PtCl62- | + 2 | Pt(IV)Cl62- |
| H3AsO3 | + 3 | H3As(III)O3 |
Main Page : – Maharashtra Board Class 11th-Chemistry – All chapters notes, solutions, videos, test, pdf.
Previous Chapter : Chapter-5-Chemical Bonding – Online Solution
Next Chapter : Chapter-7-Modern Periodic Table – Online Solution
We reply to valid query.