Redox Reactions
Maharashtra State Board-Class-11-Science-Chemistry-Chapter -6
Notes
|
Topics to be Learn :
|
Introduction :
Term Redox : Redox is an abbreviation used for the terms 'oxidation and reduction'.
A large number of phenomena such as respiration, rusting, combustion of fuel involve redox reactions.
Daily use examples :
- Apple turn brown when exposed to air : Catechol present in apple undergoes oxidation when exposed to air and imparts brown colour.
- Old car bumper changes colour : Metallic bumper undergoes oxidation by air and changes colour.
- New batteries become useless after some days : The batteries involve redox reactions during their use and consume the chemical like H2SO4. Hence they become useless after some days.
Classical ideas of redox reactions :
Classically oxidation refers to combination of an element or a substance with oxygen.
Example :
Oxidation of carbon : C (s) + O2 (g) → CO2 (g)
Oxidation of magnesium : 2Mg + O2 → 2MgO(s)
In above reactions the carbon and magnesium are oxidized on reacting with oxygen.
Classically reduction refers to combination of an element or a substance with hydrogen or removal of oxygen from its compound.
Example :
2H2 (g) + O2 (g) → 2H2O (g)
In this reaction, hydrogen is oxidised and oxygen is reduced.
Some more examples of oxidation and reduction : (i) 2H2S (g) + O2 (g) → 2S (s) + 2H2O (l) In this reaction there is removal of hydrogen and is also called oxidation. Here the sulfur in H2S loses hydrogen and undergoes oxidation while oxygen accepts hydrogen and undergoes reduction. (ii) 2Fe2O3 + 3C (s) → 4Fe (s) + 3CO2 (g) In the given reaction, Fe undergoes reduction since oxygen is removed while C undergoes oxidation since it combines with oxygen. (iii) 2H2S (g) + O2 (g) → 2S (s) + 2H2O (l) In the given reaction, S undergoes oxidation since hydrogen is removed while hydrogen undergoes oxidation since it combines with oxygen.
Oxidants/ Oxidising agent :
A reagent/substance which itself undergoes reduction and causes oxidation of another species is called oxidant /oxidising agent.
Example :
Mg (s) + F2 (s) → MgF2(s)
In the reaction fluorine is oxidant which oxidises Mg.
Reductant\reducing agent :
According to classical definition reductant is a substance which itself undergoes oxidation and causes the reduction of another substance.
Example :
Mg (s) + S(s) → MgS (s)
In the reaction Mg is reductant.
Explanation of oxidation and reduction with the help of electropositive and electronegative elements :
Oxidation : Combination of a substance with electronegative element or removal of electropositive element is called oxidation.
Reduction: Combination of a substance with electropositive element or removal of electronegative element is called reduction.
For example,
2Mg + O2 → 2MgO(s)
In this Mg undergoes oxidation while O undergoes reduction.
Key points: Oxidation is defined as : Reduction is defined as :
Redox reaction in terms of electron transfer :
Redox reaction can be described as electron transfer.
Example :
Mg + ½ O2 → Mg2+ + O2−
Development of charges on the species produced suggest the reactions can be written as :

When Mg is oxidised to MgO, the neutral Mg atom loses electrons to form Mg2+ in
MgO. The elemental oxygen gains electrons and forms O2− in MgO.
Each of the above steps represents a half reaction which involves electron transfer (loss or gain). Sum of these two half reactions or the overall reaction is a redox reaction.
Consider the following half reactions.
Fe(s) → Fe2+(aq) + 2e− ……(1)
Cu2+ (aq) + 2e− → Cu(s) ……(2)
Fe(s) + Cu2+ (aq) → Fe2+ (aq) + Cu(s) ……(3)
In the half reaction (1) Fe acts as a reducing agent.
In half reaction (2) Cu2+ acts as oxidising agent which accepts electrons.
The half reaction involving loss of electrons is called oxidation reaction and that involving gain of electrons is called reduction.
Thus eq. (1) is oxidation, eq. (2) is reduction and eq. (3) is a redox reaction.
Key points :
- Oxidant / oxidizing agent : A reagent / substance which itself undergoes reduction and causes oxidation of another species is called oxidant or oxidizing agent. This is an electron acceptor.
- Reductant / Reducing agent : A reagent / reducing agent is defined as a substance / reagent which itself undergoes oxidation and brings about reduction of another species. A reductant is electron donor.
Displacement reaction :
This is a redox reaction in which an ion (or an atom) in a compound is replaced by an ion (or an atom) of another element.
For example,
X + YZ → XZ + Y
Fe + CuSO4 → FeSO4 + Cu
Q. Identify oxidising and reducing agents involved in the following displacement reactions.
(i) Zn (s) + Cu2+(aq) → Zn2+(aq) + Cu(S)
(ii) Cu(s) + 2Ag+(aq) → Cu2+(aq) + 2Ag(S)
(iii) 2Co (s) + 3Ni2+ → 2Co3+(aq) + 3 Ni (s)
(i) Zn (s) + Cu2+(aq) → Zn2+(aq) + Cu(S) In the reaction Zn is a reducing agent and Cu2+ is an oxidising agent. (ii) Cu(s) + 2Ag+(aq) → Cu2+(aq) + 2Ag(S) In the reaction Cu is a reducing agent and Ag+ is an oxidising agent (iii) 2Co (s) + 3Ni2+ → 2Co3+(aq) + 3 Ni (s) In the reaction Co is a reducing agent and Ni2+ is an oxidising agent.
Oxidation number or oxidation state : The oxidation number (or oxidation state) of an atom in a molecule or an ion is defined as the number of charges it would carry if the electrons were completely transferred.
Explanation :
- The oxidation number or oxidation state does not always imply ionic charges on the species.
- The oxidation number of monoatomic ion is equal to charge of the ion. For example, Na+ has + 1 charge while Cl− has −1 charge, Ca2+ has + 2 charge and so on (But the oxidation states are 1+ , 1−, 2+ respectively).
- In case of a neutral molecule, the sum of the oxidation numbers of all the constituent atoms is always zero.
- The charge on a polyatomic ion is equal to the algebraic sum of the oxidation numbers of all the constituent atoms of the ion.
- From the change in the oxidation numbers in a redox reaction, an oxidant and a reductant can be identified.
Remeber : Oxidation number or oxidation state is represented as 1+, 2+, etc. while charge is represented as +1, +2, etc.
Rules to assign oxidation number : The following rules have been formulated to determine the oxidation number of an element in a compound. Rule 1. The oxidation number of each atom of an element in free state is zero. For example : each atom in H2, Cl2, O3, S8, P4, O2, Ca, etc. has oxidation number of zero. Rule 2. The oxidation number of an atom in a monoatomic ion is equal to its charge. Thus alkali metals have oxidation number +1 in all their compounds (NaCl, KCl, etc.). Alkaline earth metals have oxidation number +2 in all their compounds (CaCO3, MgCl2, etc.). Al is considered to have +3 as its oxidation number in all its compounds. Rule 3. The oxidation number of O is usually −2 in all of its compounds except in peroxide or peroxide ion where it has oxidation number of −1 and in superoxide each oxygen has oxidation number −1/2. In OF2 oxidation number of oxygen is +2. Rule 4. The oxidation number of H atom is either +1 or −1. When the H atom is bonded to nonmetals, its oxidation number is +1. When it is bonded to metals, it possesses oxidation number of −1. Rule 5. The oxidation number of F is −1 in all of its compounds. The other halogens Cl, Br and I usually exhibit oxidation number of −1 in their halide compounds. However in compounds in which halogens Cl, Br and I are bonded to oxygen, oxidation number of halogens is +1 For example, Rule 6. The algebraic sum of oxidation numbers of all the atoms in a neutral molecule is zero. Rule 7. The algebraic sum of oxidation numbers of all the atoms in a polyatomic ion is equal to net charge of the ion. Rule 8. When two or more atoms of an element are present in molecule or ion, oxidation number of the atom of that element will be average oxidation number of all the atoms of that element in that molecules. Rule 9 : The oxidation number may be zero, positive, negative, integral or fractional. By applying rules 1 to 5 it is possible to determine oxidation number(s) of atoms of various elements in molecules or ions. For doing this the rules 6, 7, 8 and 9 are useful.
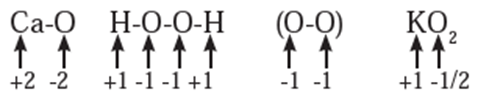
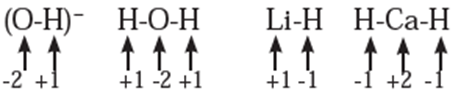
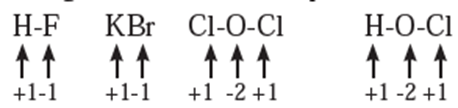
Stock notation : Oxidation number represents the oxidation state of an atom and is also denoted by Roman numeral in parentheses after the chemical symbol of the concerned element in the molecular formula.
This representation is called Stock notation after the German Scientist Alfred Stock.
Example :
- Au1+ Cl1− → Au(I)Cl
- Au3+Cl31− → Au(III)Cl3
Redox reaction in terms of oxidation number :
Oxidation : An increase in the oxidation number of an element in a given substance.
Reduction : A decrease in the oxidation number of an element in a given substance.
Oxidizing agent : A substance which increases the oxidation number of an element in a given substance, and itself undergoes decrease in oxidation number of a constituent element.
Reducing agent : A substance that lowers the oxidation number of an element in a given substance, and itself undergoes an increase in the oxidation number of a constituent element in it.
| Know This :
Some elements in a particular compound may possess fractional oxidation number. For example : C3O2, Br3O8, Na2S4O6, C8H18, etc. In these compounds oxidation number of C, Br, S, C are 4/3, 16/3, 2.5, 9/4, respectively. These oxidation numbers are actually the average oxidation number of all the atoms of elements in that compound. Different atoms of the element in such species exhibit different oxidation states. For example : Tertra thionate ion has two S atoms with oxidation number +5 and two with zero (0). Therefore, the average oxidation number of S in these species is 10/4 = 2.5
|
Balancing of redox reactions :
Two methods are used to balance chemical equation for redox processes :
- Oxidation number method
- Half reaction method or Ion electron method.
The Oxidation number method : Step 1 : Balance the given equation for all atoms except H and O. Identify the atoms undergoing change in oxidation number. Step 2 : Show an increase in oxidation number for oxidised species and a decrease in oxidation number for reduced species. Balance total increase and decrease in oxidation numbers. Step 3 : Balance O atoms by adding H2O to the side deficient of O atoms and balance H atoms by adding H+ ions to the side deficient of H atoms and they are finally removed by forming H2O. Step 4 : In basic medium OH− ions are added which are removed as H2O. Step 5 : Check the equation with respect to number of atoms of each element on both the sides.
Half reaction methods : Step 1 : Assign oxidation numbers to all atoms in the given reaction. Step 2 : Divide the equation into two half equations, one for oxidation and the other for reduction. Step 3 : Find the loss of electrons and gain of electrons by atoms in the reaction. Step 4 : Balance the loss and gain of electrons. Step 5 : Add both the reactions. Step 6 : For acidic medium H+ ions are added while for basic medium OH− are added.
Redox reaction and electrode potential :
Redox reaction with the help of Daniel cell :
Consider the following displacement reaction,
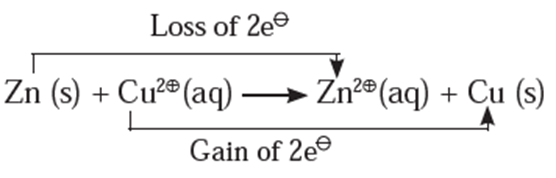
The electron transfer from Zn atom to Cu2+ ions can be demonstrated with the help of Daniel Cell reactions.
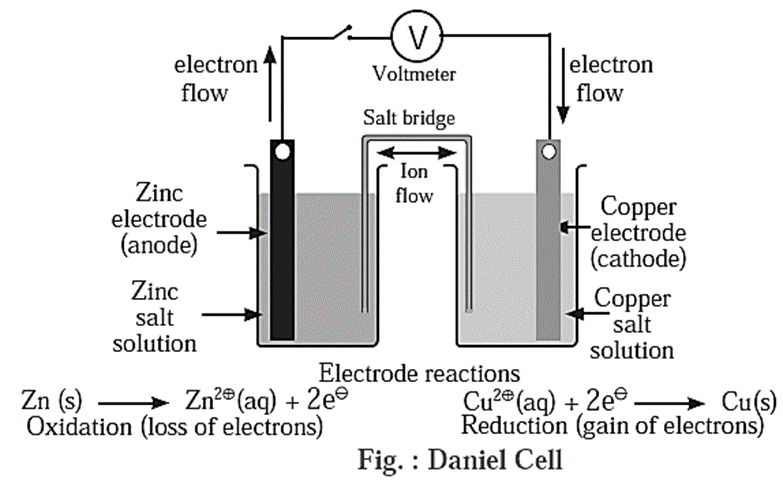
Construction :
- Daniell cell consist of two half cells. One half cell consists of a beaker containing ZnSO4 (1 M) solution in which a polished strip of metallic zinc is immersed while the second half cell consists of a beaker containing CuSO4 (1 M) solution in which a polished strip of metallic copper is immersed.
- Two half cells are electrically connected by a U-shaped salt bride containing a gel of KCl or NH4NO3 in agar-agar.
Working of Daniell cell : When the circuit is complete, following reactions take place :
At zinc electrode : Zinc atoms from zinc plate lose electrons spontaneously which flow in the external circuit from zinc plate to copper plate through wire.
Zn(s) → Zn2+(aq) + 2e− ..(oxidation)
At copper electrode :
- Cu2+ from solution receive these electrons through copper plate and are reduced to copper atoms which are deposited on the copper plate.
Cu2+(aq) + 2e− → Cu(s) …(reduction)
- The net reaction in the cell is an electrochemical redox reaction represented as,
Zn(s) + Cu2+(aq) → Zn2+(aq) + Cu(s)
- In the cell zinc electrode is an anode while copper electrode is a cathode. In the external circuit electrons flow from anode (Zn) to cathode (Cu). An electrical potential called electrode potential is established at two electrodes of the electrochemical cell.
Electrode potential :
An electrical potential established at the electrode of an electrochemical cell is called an electrode potential. The magnitude of the electrode potential depends upon the nature of metal and ions, concentration of ions and temperature.
Electrode reaction :
The reaction associated with an electrode involving metal and ions is called electrode reaction.
Redox couple :
The two chemical species which are linked by transfer of electrons represents a redox couple. For example, Zn(s) | Zn2+(aq)
Standard electrode potential : The observed electrode potential is referred to as standard electrode potential (E0) when the concentration of each species involved in the electrode reaction is unity and the temperature is 298 K.
Significance of standard electrode potential, E0 :
Q. On the basis of standard electrode potential explain :
(a) the reducing power of alkali metals and
(b) oxidising power of fluorine.
(a) Since redox couple of alkali metals has larger negative value for standard reduction potential, they have a great tendency to give away electrons and form cations. Na (s) → Na+ + e− E0 = −2.71 v Therefore alkali metals are strong reducing agents. (b) Since redox couple of fluorine has very large positive value for standard potential, it has a great tendency to gain electron and form anion. F2(g) + 2e− → 2F− E0 = +2.87 v Therefore fluorine is a strong oxidising agent.
Standard electrode protentianls of some redox couples :

Main Page : – Maharashtra Board Class 11th-Chemistry – All chapters notes, solutions, videos, test, pdf.
Previous Chapter : Chapter-5-Chemical Bonding – Online Notes
Next Chapter : Chapter-7-Modern Periodic Table – Online Notes
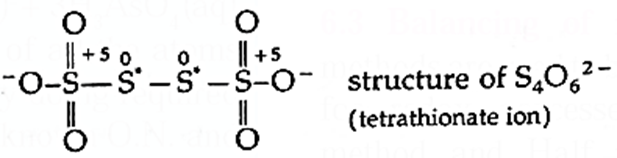
We reply to valid query.