Basic Analytical Techniques
Class-11-Science-Chemistry-Chapter -3 Maharashtra State Board
Notes
|
Topics to be Learn :
|
Introduction
- There has been a systematic development in the techniques used for analysis of chemical substances.
- Chemical substances occur in nature in impure stage.
- When chemical substances synthesized in the laboratory they are obtained in crude and impure form.
- Before investigating their composition and properties it is essential to obtain them in the pure form.
- Thus, methods for purification and separation are used in the analysis of chemical substances.
- These methods depend upon the differences in physical properties such as solubility in a given solvent, boiling point, etc.
Purification of solids
A solid substance may contain two types of impurities, those
- Impurities which are soluble in the solvent in which the main substance is soluble.
- Impurities which are not soluble in the solvent in which the main substance is soluble.
- The impurities which are not soluble in the solvent can be separated easily by a simple process called filtration
Filtration : The process of separating undissolved impurities from a liquid by a simple process by passing through a filter is called filtration.
Process of separating a mixture of sand and water by simple filtration :
A mixture of sand and water can be separated by the process of filtration. (see Fig.)
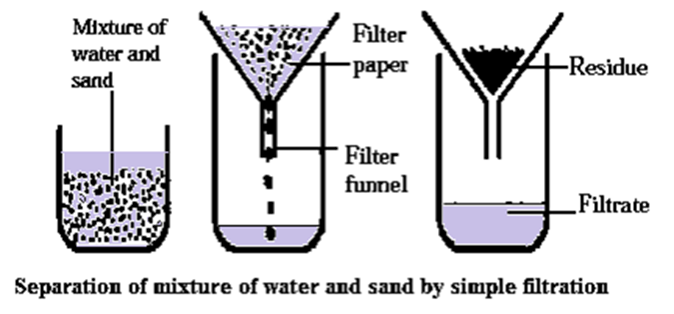
- A circular piece of filter paper is folded to form a cone and fitted in the funnel.
- The funnel is fixed on a stand and a beaker is kept below it.
- The paper is moistened before filtration.
- The mixture of sand and water is poured on the filter paper.
- The insoluble part remaining on the filter paper after filtration is called residue.
- The liquid passes through the filter paper and collected in the beaker is called filtrate.
Note : A moistened filter paper sticks to the funnel preventing any solid from escaping from the sides of the funnel.
Filtration under suction : When filtration is carried out using a vacuum pump it is called filtration under suction. It is a faster and more efficient technique than simple filtration.
Setup used for the process of filtration under suction :
- A thick walled conical flask with a side arm is used for the process of filtration under suction.
- A rubber tubing is fitted to the side arm which is connected to the safety bottle. This prevents the sucking of the filtrate into the suction pump.
- A special porcelain funnel called the Buchner funnel is fitted on the conical flask with the help of a rubber cork (rubber bung).
- The Buchner funnel has a porous circular bottom. A circular filter paper of correct size is placed on the circular porous bottom of the Buchner funnel.
- The filter paper is moistened with a few drops of water or solvent.
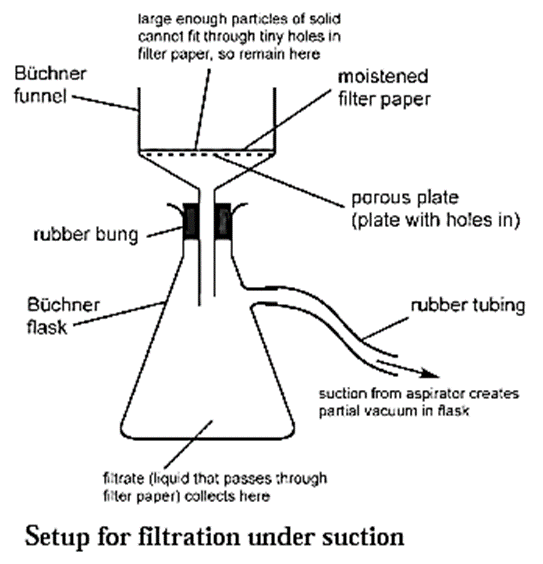
Process for filtration under suction :
- The Buchner funnel is fitted on the conical flask with the help of the rubber cork (rubber bung).
- The filter paper fitted on the Buchner funnel is first moistened with water or a solvent, so that it remains fixed on the funnel.
- The solution to be filtered is poured on the filter paper.
- The vacuum pump is started to create suction.
- The insoluble part in the solution remains on the filter paper. This is called the residue.
- The liquid collects in the flask. This is called the filtrate.
Crystallization : The method of crystallization is used to purify impure solid substances.
- The technique of crystallization is used for the purification of solid organic compounds.
- When a crude solid is made of mainly one substance and has some impurities, it is purified by the process of crystallization.
It is done in four steps:
- (i) Preparation of saturated solution
- (ii) Hot filtration
- (iii) Cooling of the filtrate
- (iv) Filtration.
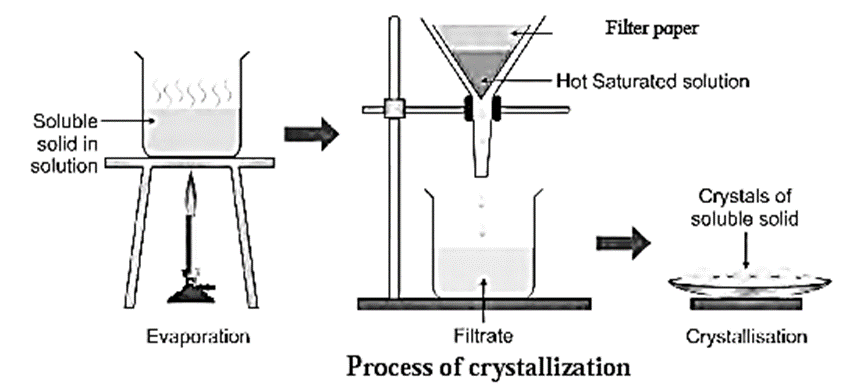
(i) Preparation of saturated solution :
- A small but sufficient quantity of a suitable solvent is added to the crude (impure) solid and stirred. The crude solid forms a solution.
- The solution is then boiled. The main substance (solute) after dissolving forms a saturated solution with respect to the soluble impurities which are present in small proportion.
(ii) Hot filtration
- The above solution is quickly filtered while hot.
- Filtration under suction allows rapid filtration.
- Undissolved impurities get removed in this process as residue.
(iii) Cooling of the filtrate :
- The hot filtrate is allowed to cool. Solubility of a substance decreases with lowering of temperature.
- The excess quantity of the dissolved solute comes out of the solution in the form of crystals.
- The dissolved impurities continue to stay in the solution in dissolved state even on cooling.
- The separated crystals are, therefore, free from soluble impurities.
(iv) Filtration :
- The crytals of the pure substance are separated by filtration. The filtrate obtained is called mother liquor.
- The crystals so formed are free from soluble as well as insoluble impurities.
Properties of the solvent :
The solvent to be used for crystallization must have following properties :
- The compound to be crystallized should be least or sparingly soluble in the solvent at room temperature but highly soluble at high temperature.
- Solvent should not react chemically with the compound to be purified.
- Solvent should be volatile so that it can be removed easily.
Solvents : Water, ethyl alcohol, methyl alcohol, acetone, ether or their combinations are generally used as solvent for crystallization.
Impure sample of common salt, impure copper sulphate and benzoic acid can be purified by crystallization using water as a solvent.
Process used to purify an impure sample of common salt :
Many times the common salt obtained from the market may contain some siliceous matter and other impurities. These can be removed and larger crystals of pure NaCl can be obtained.
Common salt can be crystallized from the impure sample by the process of crystallization.
- Take some water in a beaker and add some amount of salt in it. Stir the solution with a glass rod. Add more salt till no more salt dissolves in it giving a saturated solution.
- Heat the solution.
- Filter the hot solution to remove the insoluble impurities. '
- Collect the filtrate in an evaporating dish and allow it to cool.
- Crystals of pure salt (NaCl) separate out leaving soluble impurities in the mother liquor.
- Filter the solution and collect the crystals on the filter paper and dry them.
Fractional crystallization :
Fractional crystallisation is a process wherein two or more soluble substances having widely different solubilities in the same solvent at room temperature are separated by crystallization.
Process of fractional crystallization :
- Consider a mixture of two solutes A and B. The mixture is first dissolved in a suitable hot solvent to prepare a saturated solution.
- The saturated solution is filtered to remove dust particles and then allowed to cool.
- As the solution cools, the solute which is less soluble crystallizes out first.
- The crystals are filtered, washed with the solvent and dried to obtain one of the solutes in the mixture.
- The mother liquor is then concentrated by evaporating the solvent. The crystals of the second solute crystallize out.
- These crystals are filtered to obtain the purified second solute.
Distillation :
Distillation is an important method used to separate.
- Volatile liquids from non-volatile impurities
- Liquids having sufficient difference in their boiling point.
Simple distillation : Liquids which boil without decomposition at atmospheric pressure and which contain nonvolatile impurities are purified by the process of simple distillation.
Setup used for simple distillation :
The apparatus used for simple distillation is shown in Fig.
- It consists of a round bottom flask fitted with a cork having a thermometer in it.
- The flask is fixed by a clamp and is placed on a water bath or oil bath or sometimes on a wire gauze kept on a stand.
- The flask has a side arm which is connected to a condenser. The condenser has a jacket with two outlets through which water is circulated.
- The condenser is connected to a receiver to collect the purified liquid.
- The thermometer is placed in such a way that the bulb of the thermometer is just below the side arm of the round bottom flask.
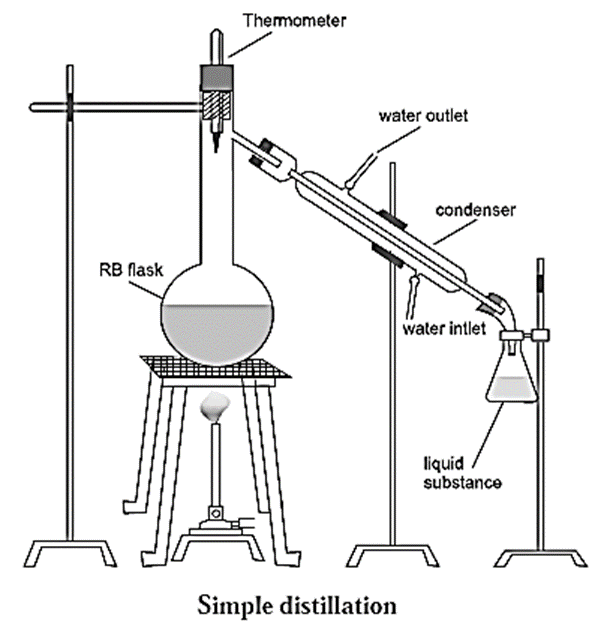
Process of simple distillation :
- The liquid to be purified is taken in the round bottom flask.
- The flask is heated.
- When the temperature of the liquid reaches its boiling point, the liquid starts boiling.
- At this point, the vapours rise up and pass through the side arm of the flask and go into the condenser.
- Cold water is circulated through the jacket of the condenser which cool the vapours of the liquid.
- The vapours condense to form the liquid which is collected in the receiver.
Method used to separate the mixture of acetone and water :
- The mixture of water and acetone is separated by the process of distillation. The mixture is taken in a distillation flask and heated carefully on a water bath.
- When the temperature reaches 56 °C, acetone distills out which can be collected in one receiver.
- After all the acetone has distilled out, the receiver has to be changed.
- A little amount of the liquid has to be discarded to ensure that there are no traces of acetone left in the tube of the condenser.
- When the temperature reaches 100 °C, water distills out, which can be collected in another receiver.
Principle used to separate a mixture of acetone and water :
- A mixture of acetone and water can be separated by the method of simple distillation as the liquids have a wide difference in their boiling points
- Acetone and water are two miscible liquids. The boiling point of acetone is 56 °C and that of water is 100 °C.
- When the mixture is heated and the temperature reaches 56 °C, which is the boiling point of acetone, then only acetone distills out.
- When the temperature rises to 100 °C, i.e the boiling point of water, then water distills out and is collected separately.
Fractional Distillation :
If in a mixture the difference in boiling points of two liquids is not appreciable, they cannot be separated from each other using the simple distillation assembly.
The process of separating the components of a mixture of two or more miscible liquids (which do not differ much in their boiling points) is known as fractional distillation.
Difference between assembly used for fractional distillation and that used in simple distillation :
The assembly used in fractional distillation is similar to the assembly used in distillation, except that in this setup the distillation flask is fitted with a fractionating column (see Fig.).
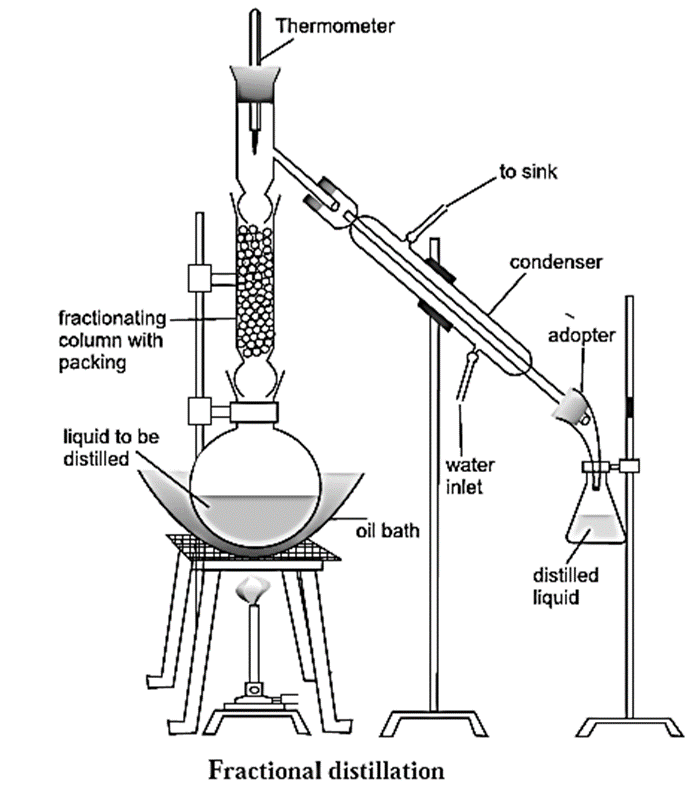
Fractional distillation method :
Fractional distillation method used to separate a mixture of two liquids :
- Consider a mixture of two liquids (A) and (B) having boiling points 363 K and 373 K respectively. The two liquids have very close boiling points hence they have to be separated by the method of fractional distillation.
- When the mixture is heated, both liquid (A) along liquid (B) form vapours.
- Since, liquid (A) is more volatile compared to liquid (B), vapours of liquid (A) along with little of liquid (B) rise up and come in contact with the large surface of the fractionating column.
- Vapours of liquid (B) condense and fall back into the distillation flask.
- As they fall back, there is an exchange between the ascending vapours and the descending liquid.
- The ascending vapours of (B) are scrubbed off by the descending liquid (B), thus making the vapours richer in (A).
- This process is repeated each time the vapours and the liquid come in contact with the fractionating column.
- The rising vapours become rich in (A). When the temperature at the top of the fractionating column reaches 363K, which is the boiling point of the liquid (A), the vapours of liquid (A) pass through the condenser, get cooled and are collected in a receiver.
- The liquid in the distillation flask is richer in (B). The process is repeated to further purify the separated components.
Function of a fractionating column :
The types of fractionating columns used in fractionating distillation are (1) Simple packed column and (2) Bubble plate column.

- A fractionating column is a vertical glass tube filled with glass beads. The glass beads provide a large surface area for hot vapours to cool and condense repeatedly.
- Vapours of the more volatile liquid with lower boiling point rise up more than the vapours of liquid having higher boiling point and pass over into the condenser.
- Two miscible liquids with their boiling points close to each other can be separated using fractionating column.
- Thus, a fractionating column is an arrangement for providing different temperature zones inside the column during distillation. The highest temperature is at the bottom of the column and the lowest temperature is near its top.
Distillation under Reduced Pressure :
Liquids having very high boiling point or those which decompose on heating are purified by carrying out distillation under reduced pressure.
- Principle : Lower the external pressure lower is the boiling point of the liquid.
- Explanation : Hence a liquid is made to boil at a temperature lower than its normal boiling point by reducing the pressure on the surface of the liquid. Pressure is reduced by using a water pump or vacuum pump;
- Application : In soap industry glycerol is separated from soap by using this technique.
Solvent Extraction
When an organic substance is present in an aqueous solution, it can be extracted from that solution by shaking it with an organic solvent in which the substance is more soluble.
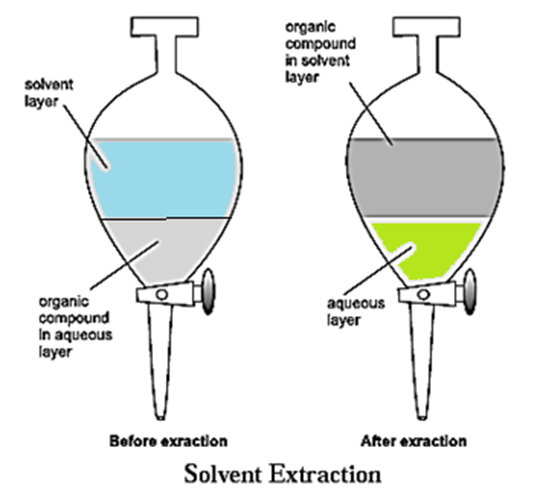
- The process of solvent extraction depends on the difference in solubility of the solute in two immiscible liquids.
- This process is carried out using a separating funnel. (see Fig.)
- The solute distributes itself between the two immiscible liquids.
- On shaking the solution for a few times with small volumes of the organic phase, most of the solute gets extracted into the organic phase.
- The organic solvent is then removed by distillation and the solute is collected.
Properties of solvents for solvent extraction :
- The organic solvent selected should be such that the organic compound (solute) to be separated is more soluble in it.
- The organic solvent should be immiscible with water.
- The organic solvent should not chemically react with the organic compound (solute) present in the aqueous solution.
- The density of the solvent must be different than that of the aqueous phase which facilitates the quick settling of the two layers.
Advantages of solvent extraction :
- Solvent extraction involves the use of a simple apparatus like a separating funnel.
- This technique helps in obtaining clean separations in a short span of time.
Continuous extraction :
- This technique is used if the solute to be extracted is less soluble in the organic phase.
- The organic solvent is continuously distilled within the same assembly.
- Thus, the same amount of the organic solvent can be used repeatedly for extraction.
- This technique has an advantage of using small quantities of the organic solvent.
Chromatographic techniques :
Chromatography is a technique used to separate components of a mixture, and also purify compounds. The Greek word Chroma meaning Colour.
In 1903, Tswett discovered this technique for separating the coloured components found in plants.
Principle : Similar to solvent extraction i. e. distribution of the solutes in two phases.
In chromatography two phases for separation used are (a) Stationary phase and (b) Mobile phase.
- The separation of substances in chromatography is based on the difference in the rates at which different substances in the mixture move through the stationary phase under the influence of the mobile phase.
- The mixture of the substances is loaded at one end of the mobile phase. Then the mobile phase which is a pure solvent or mixture of solvents is allowed to move slowly over the stationary phase.
- Different substances of the mixture show different affinities towards the stationary phase and the mobile phase.
- Depending upon the relative affinities of the components, the substances are either retained on the surface of the stationary phase or they move along the mobile phase and are gradually separated.
Stationary phase : The stationary phase is a solid or liquid supported on a solid which remains fixed in a place. The substances to be separated are selectively adsorbed or retained on the stationary phase.
Depending on the stationary phase chromatography is classified into two types :
- Adsorption chromatography
- Partition chromatography
Adsorption chromatography :
Principle of adsorption chromatography : This type of chromatography is based on the principle of differential adsorption. Different solutes are adsorbed to different extent on the stationary phase depending upon their ability of adsorption.
Adsorption chromatography is further classified into two types :
- Column chromatography
- Thin layer chromatography
(i) Column chromatography :
- This type involves the separation of components over a column of stationary phase.
- The stationary phase material can be Alumina, Silica gel.
Principle of Column chromatography : Column chromatography is based on the principle of Differential adsorption.
Process of column chromatography :
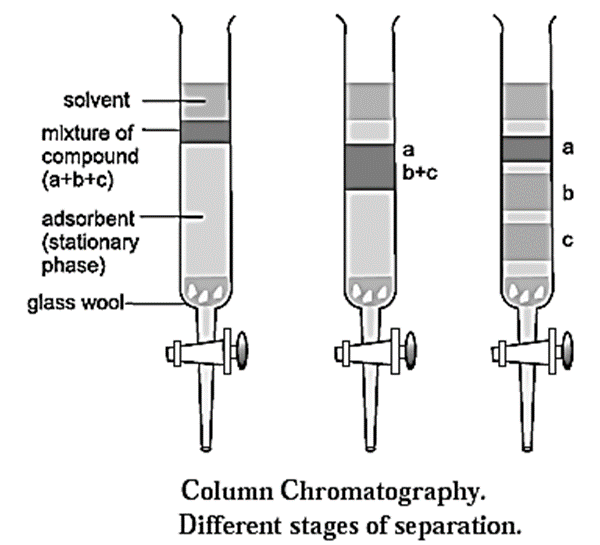
- A slurry of stationary phase material like alumina or silica gel is filled in a long glass tube provided with a stop cock at the bottom and a glass wool plug at the lower end.
- The mixture to be separated is dissolved in a small amount of an appropriate solvent and loaded on top of the adsorbent column.
- A suitable mobile phase is poured over the adsorbent column. The mobile phase could be a single solvent or a mixture of solvents.
- As the mixture moves down the column slowly, the different solutes in the mixture get adsorbed at different regions on the column depending upon their ability of adsorption. This results in the formation of different bands on the column. (see Fig.)
- The different solutes can be desorbed and collected separately in different containers by adding more solvent at the top, by the process of elution.
- The solute which is adsorbed less strongly is desorbed first and is eluted first, while the solute which is adsorbed strongly is eluted later.
- The solutions of the different solutes are collected separately. The solutions are then evaporated to obtain the pure solutes. Thus a mixture can be separated and identified by column chromatography.
(ii) Thin Layer Chromatography (TLC) :
- Thin layer chromatography is a type adsorption chromatography where separation takes place over a glass plate coated with a thin layer of adsorbent material which acts as the stationary phase.
- A thin layer (0.2 mm thick) of adsorbent like silica gel or alumina is spread over a glass plate. This plate is called the chromplate or TLC plate.
Principle of TLC : Thin layer chromatography is based on the principle of Differential adsorption.
Techniques of thin layer chromatography :
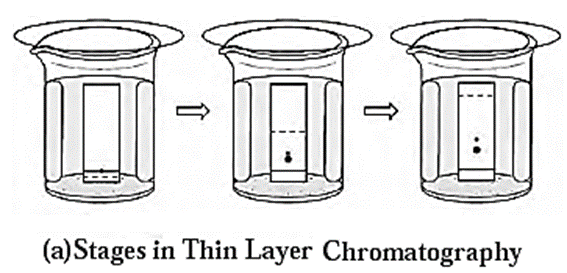
- On a thin glass plate (chromplate) a slurry of solid adsorbent like silica gel or alumina is spread and allowed to dry. This plate acts as a stationary phase.
- With the help of capillary, solution of mixture to be separated is spotted on the chromplate as a small spot at about 2 cm from one end of the plate.
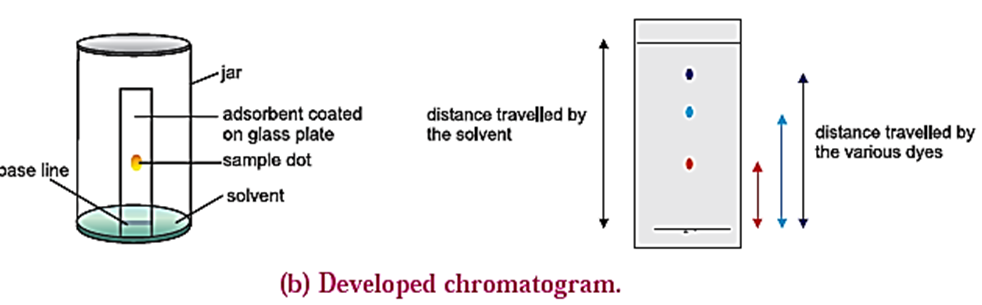
- The plate is then placed in a closed jar containing the mobile phase (eluant). Closing of the jar ensures that the jar is saturated with the solvent vapour. Care should be taken that the spot is above the level of the mobile phase.
- As the solvent of the mobile phase travels along the plate, the different components of the mixture also move along with it.
- The different components move at different distances depending upon their degree of adsorption and thus get separated. [see Fig.(a)]
- Coloured components appear as different coloured spots on the plate. [see Fig (b)]
- If the components are not colored but have property of fluorescence they can be visualised under UV light, or the plate can be kept in a chamber containing a few iodine crystals.
- The Iodine vapors are adsorbed by the components and the spots appear brown.
- Amino acids are visualised by spraying the plate with a solution of ninhydrin. This is known as spraying agent.
Partition Chromatography :
Principle of partition chromatography : In this type of chromatography, the separation of the components takes place by the continuous differential partitioning of the components between the stationary and mobile phase. For example, Paper Chromatography.
Paper Chromatography :
- In this technique a special quality paper, Whatmann paper number 1, is used for separation.
- The water trapped in the fibres of the paper acts as the stationary phase.
Principle of Paper chromatography :Paper chromatography is based on the principle of Differential partitioning of components of a mixture between a stationary phase and a mobile phase.
Techniques of paper chromatography :
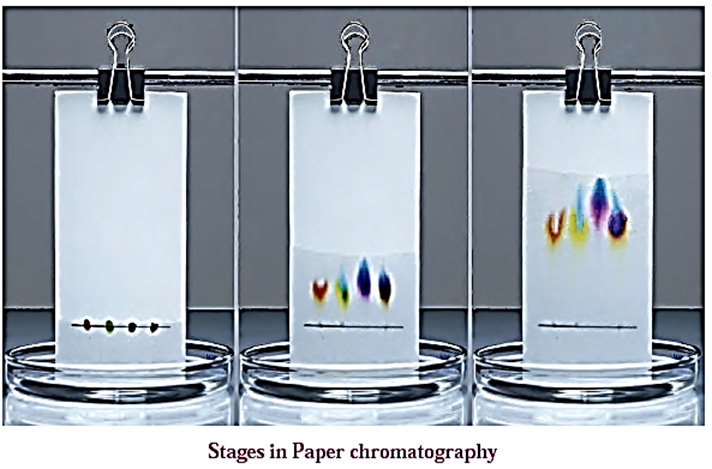
- The solution of mixture to be separated is spotted on the strip of the chromatography paper at about 2 cm from one end of the paper using a glass capillary.
- The paper is then suspended in a chamber containing the mobile phase. Care should be taken that the spot is above the level of the mobile phase.
- As the mobile phase rises along the paper due to capillary action, it flows over the spot. The different solutes in the mixture are partitioned between the stationary phase (water) and the mobile phase. (see Fig.)
- Different solutes are retained differently on the paper depending upon their selective partitioning between the two phases.
- The paper (chromatogram) is removed from the solvent before the solvent reaches the upper end and dried.
- The components are separated and their spots are visible at different heights on the paper.
- The developed paper strip is called the chromatogram.
Similar to TLC (Thin layer chromatography) the colored components are visible as colored spots and the colourless components are observed under UV light or using a spraying agent.
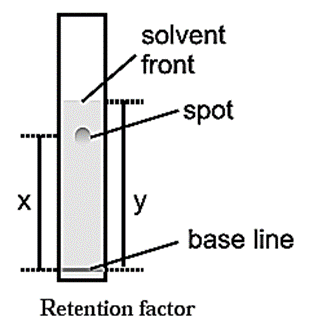
Retention factor (Rf) : Migration of the solute relative to the solvent front gives an idea about the relative retention of the solutes on the stationary phase. This is termed as the Rf of the solute.
\(R_f=\frac{\text{Distance travelled by the solute from the base line}}{\text{Distance travelled by the solvent from the base line}}\)
Main Page : – Maharashtra Board Class 11th-Chemistry – All chapters notes, solutions, videos, test, pdf.
Previous Chapter : Chapter-2-Introduction to Analytical Chemistry – – Online Notes
Next Chapter : Chapter-4-Structure of Atoms – Online Notes
We reply to valid query.