Metals and Non-Metals
Class-10-CBSE-NCERT-Science-Chapter-3
Notes Part-1
|
Topics to be learn : Part-1
|
Physical Properties :
Metals : Metals are the elements which can easily form positive ions by losing electrons.
- Example : Magnesium (Mg) is a metal which forms positive ions, Mg2+ by losing 2 electrons.
- Copper, silver, gold, aluminium are some of the metallic elements.
Important physical characteristics of metals :
- Conduction of heat and electricity: Metals are good conductors of heat and electricity.
- Metallic lustre: Metals have shining surface.
- Strength: Metals have high tensile strength.
- Melting points and boiling points: Except sodium and potassium, metals have very high melting points and boiling points. Iron melts at 155-39°C.
- Malleability and ductility: Metals can be drawn into thin wires (ductile) and can also be beaten into thin sheets with a hammer (malleable).
- Densities: Except sodium and potassium, metals are quite hard and have high densities.
- Hardness : Hardness of a substance is its ability to resist cutting, scratching and grinding. Iron, aluminium and lead are hard metals.
Uses of Metals and their physical properties :
- Some metals are used for making cooking vessels because these (i) are good conductors of heat (ii) have high melting points (iii) are non-corrosive (iv) are easily available.
- Aluminium or copper is used for making electrical wires because these are good conductors of electricity. (They are coated with rubber like material because when in circuit, there is a current flowing through wire and if you touch the exposed portion, it does not give you a shock)
- The metal Gallium has very low melting point and can melt with heat of our palm.
- Gold and silver are highly malleable and ductile and have brilliant shining. So are used to make jewellery.
- Tungsten used almost exclusively for filament of electric lamps. Tungsten on passing current gets heated and glows.
- Sodium and potassium are very reactive and react with air even at room temperature. So are kept in kerosene.
Non-metals : Non-metals are the elements which form negative ions by gaining electrons.
- Example : Oxygen is a non-metal which forms oxide ions, O−2, by gaining electrons.
- Hydrogen, carbon, silicon etc. are some of the non-metallic elements.
Non-metals and their types :
- There are 22 non-metals
- Ten non-metals are solids.
- Important solid non-metals are: Boron (B), Carbon (C), Silicon (Si), Phosphorus (P), Arsenic (As), Sulphur (S), Iodine (I).
- Eleven non-metals are gases. These are: Hydrogen (H), Nitrogen (N), Oxygen (O), Fluorine (F), Neon (Ne), Chlorine (Cl), Helium (He), Argon (Ar), Krypton (Kr), Xenon (Xe), Radon (Rn).
- One non-metal is a liquid. For example, bromine (Br).
- Samples of carbon, sulphur and iodine do not show the properties of metals. However, carbon in the form of graphite is a good conductor of electricity and iodine has a shining surface.
Metalloids : Arsenic and antimony are metalloids. They are called metalloids because they exhibit characteristics of both metals and non-metals.
The important physical properties of non-metals are given below:
- Non-metals are brittle, i.e., they cannot be beaten into sheets. When hammered, they break into pieces. For example, sulphur and phosphorus are brittle non-metals.
- Non-metals are non-ductile, i.e., they cannot be drawn into thin wire on stretching.
- Non-metals are bad conductors of heat and electricity: Except carbon (in the form of graphite) non-metals do not conduct heat and electricity because unlike metals they have no free electrons.
- Non-metals are dull: Except iodine and graphite, non-metals have no lustre (shine).
- Non-metals have comparatively low melting and boiling pts.
- Non-metals have low densities.
- Non-metals may be solid, liquid or gas at room temperature. Carbon, sulphur and phosphorus are solid non-metals, bromine is a liquid non-metal; hydrogen, oxygen and nitrogen are gaseous non-metals.
- Most-solid non-metals are soft: Only carbon (in the form of diamond) is very hard.
- Non-metals are not strong; i.e., these have low tensile strength.
Q. Why is it not possible to group metals and non-metals on the basis of their physical properties? Explain.
We cannot group metals and non-metals on the basis of their physical properties only because there are many exceptions both in metals and non-metals. For example,
- All metals except mercury are solids.
- Metals have generally high melting points but gallium and caesium have very low melting points.
- Metals are hard but alkali metals like lithium, sodium and potassium are so soft that they can be cut with a knife.
- Non-metals are dull but iodine is lustrous.
- Non-metals are bad conductor of electricity but carbon in the form of graphite is a conductor of electricity.
- Most solid non-metals are soft but carbon in the form of diamond is the hardest natural substance.
Differences in the physical properties of metals and non-metals :
| Property | Metal | Non-metal |
| Physical state | All metals are solids except mercury which is a liquid. | Non-metals exist in all three states. H2, O2, N2 are gases; bromine is a liquid; carbon, sulphur and phosphorus are solids. |
| Melting and boiling points. | Most of the metals have high melting and boiling points. | The melting and boiling points of non-metals are comparatively low. |
| Lustre | Metals show bright metallic lustre. | These are generally dull except iodine. |
| Malleability | Malleable, i.e., can be beaten to thin sheets or foils. | Not malleable. |
| Ductility | They are ductile i.e., can be drawn into wires. | Not ductile.
|
| Conductivity | They are good conductors of heat and electricity. | They are bad conductors of heat and electricity (except graphite). |
Chemical Properties of Metals :
Some important chemical properties of metals :
(i) Reaction with oxygen:
- Most of the metals react with oxygen of air and form their oxides.
- Most metal oxides are insoluble in water but some of these dissolve in water to form alkalies.
- The spontaneity with which a metal reacts with oxygen depends on the chemical reactivity of the metal.
- Some metals react with oxygen even at room temperature, some react on heating, whereas still others react only on prolonged heating.
Example :
(a) Sodium reacts with oxygen at room temperature to form sodium oxide (Na2O). This is a basic oxide and dissolves in water to form sodium hydroxide, an alkali.
4Na + O2 → 2Na2O
Na2O + H2O → 2NaOH (Sodium hydroxide)
(b) Magnesium burns in air with a dazzling white flame once heated to a temperature called its ignition temperature. It does not react with air at room temperature.
2Mg + O2 → 2MgO (Magnesium oxide)
(c) Copper and iron metals are less reactive and react with oxygen only on prolonged heating.
2Cu + O2 → 2CuO (Cupric oxide)
4Fe + 3O2 → 2Fe2O3 (Ferric oxide)
(d) Silver and gold do not react with oxygen even at high temperature.
(ii) Reaction with water:
- More reactive metals react with Water at room temperature but less reactive metals react on heating with water or steam. Hydrogen is formed in all these cases.
Examples :
(a) Sodium and potassium react with water at room temperature very briskly and form sodium hydroxide and hydrogen gas is evolved.
2Na + 2H2O → 2NaOH + H2
(b) Magnesium and calcium react slowly with cold water but react rapidly with boiling water but metals like aluminium, iron and zinc do not react with water, they react with steam.
Mg + H2O → MgO + H2
Zn + H2O (Steam) → ZnO + H2
3Fe + 4H2O (Steam) → Fe3O4 + 4H2
(c) Metals like lead, copper, silver and gold do not react even with steam. Metal oxides which dissolve in water are called alkalis.
Know This :
|
(iii) Reaction with dilute acids:
- Many metals react with dilute acids to give metal salts and hydrogen.
- The rate of effervescence of hydrogen depends on the reactivity of the metal. Higher rate of effervescence means higher reactivity of the given metal.
- The reactions of metals with dilute hydrochloric acid and dilute sulphuric acid are similar. With dilute hydrochloric acid (HCl) they give metal chlorides and hydrogen; with dilute sulphuric acid (H2SO4), they give metal sulphates and hydrogen.
- Nitric acid is an oxidising agent, so it reacts differently.
- Mg and Mn react with dil. HNO3 to evolve H2 gas.
- Less reactive metals like copper, silver, gold do not react with dilute HCl.
Examples :
- 2Na + 2HCl → 2NaCl + H2
- Mg + 2HCl → MgCl2 + H2
Know This :
|
(iv) Reaction with hydrogen:
- The metals generally do not combine with hydrogen
- The more reactive metals such as sodium, calcium react with hydrogen to form metal hydrides.
2Na + H2 →2NaH (Sodium hydride)
Ca + H2 → CaH2 (Calcium hydride)
- Magnesium and copper form hydrides with difficulty.
(v) Reaction with halogens:
Metals react with chlorine to give metal chlorides.
2Na + Cl2 \(\underrightarrow{Heat}\) 2NaCl
Mg + Cl2 \(\underrightarrow{Heat}\) MgCl2
2Fe + 3 Cl2 \(\underrightarrow{Heat}\) 2FeCl3
(vi) Reaction with sulphur:
Metals react with sulphur to give their metal sulphides.
2Na. + S → Na2S (Sodium sulphide)
Mg + S → MgS (Magnesium sulphide)
(vii) Displacement of metals from their salt solutions:
- The more reactive metals displace the less reactive metals from their salt solutions.
- If we take a solution of copper sulphate (CuSO4) and put a strip of iron metal in this solution, the blue colour of copper sulphate solution fades gradually and copper metal is deposited on the iron strip.
Fe(s) + Cu2+SO42- → Fe2+SO42- + Cu(s)
Copper sulphate Ferrous sulphate
(Blue solution) (Green solution)
- If we dip copper wire in ferrous sulphate solution; no reaction will be observed. This means that iron is more reactive than copper.
- If we put gold or platinum in a copper sulphate solution, then copper is not displaced because both gold and platinum are less reactive than copper and do not give electrons to reduce copper ions into copper metal.
Know This :
|
Q. Reverse of the following chemical reaction is not possible, Why ?
Zn(s) + CuSO4(aq) → ZnSO4(aq) + Cu(s)
When a less reactive metal is added to a solution of more reactive metal, no reaction takes place. So reverse of above reaction or the reaction given is not possible. Cu + ZnSO4 → No reaction
Another example of no reaction : Ag + CuSO4 → No reaction
Q. “Hydrogen gas is not evolved when most metals react with nitric acid.” State reasons to justify this statement.
Hydrogen gas is not evolved when most metals react with nitric acid because
HNO3 is a strong oxidising agent. It oxidises the H2 produced to water and itself gets reduced to any of the nitrogen oxides (N2O, NO, NO2,).
The Reactivity Series :
Some metals are very reactive while others are less reactive or do not react at all.
For example,
- Sodium and potassium react very vigorously even with cold water, so they can be said to be very reactive metals.
- Zinc and iron do not react with hot water even but react with steam, so these are less reactive metals.
- Copper and silver do not react even with steam, so they are quite unreactive metals.
Similarly, metals react with acids with different vigorousity and rate of evolution of H2 is different in different cases.
As all the metals do not react with oxygen, water or acid and so their comparative reactivities cannot be ascertained.
But displacement reactions give better evidence about the comparative reactivity of one metal with respect to another. For example, if metal A displaces metal B from its solution, it is more reactive than B and so on.
The arrangement of metals in a vertical column in order of decreasing reactivities is called the activity series of metals.
Following is the activity series of common metals.
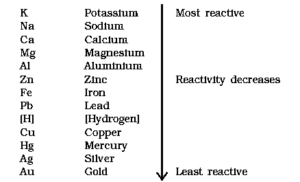
Know This :
|
Q. Carbon at red heat removes oxygen from the oxides of the metals A, B and C but not from the oxide of metal D. Metal C removes oxygen from the oxide of A but not from the oxide of B. Arrange the metals A, B, C and D in a decreasing order of reactivity. Also give reasons in support of your answer.
- Since carbon removes oxygen from the oxides of metals A, B and C, therefore, carbon is more reactive than metals A, B, C.
- D is more reactive than carbon as it (carbon) cannot remove oxygen from the oxide of D, i.e., D > carbon > A, B or C.
- Metal C is more reactive than metal A because it can remove oxygen from oxide of A, i.e., C > A.
- Since metal C cannot remove oxygen from the oxide of metal B, therefore, metal B is more reactive than C, i.e., B > C.
- Thus the decreasing order of reactivity is D > B > C > A.
Know This :
Fe(s) → Fe2+(aq) + 2e− Cu2+(aq) + 2e → Cu(s) _______________________ Fe(s) + Cu2+(aq) → Cu(s) + Fe2+
|
Chemical properties of non-metals. OR General characteristics of non-metals:
The following are the general characteristics of non-metals:
(i) Non-metals are electronegative elements: The non-metals have the tendency to accept electrons and form negatively charged ions, so non-metals are called electronegative elements. e.g., oxygen is a non-metal which gives negative oxide ions, O2− chlorine is a non-metal which gives negatively charged chloride ions, Cl−.
(ii) Reaction with oxygen: With oxygen non-metals form acidic oxides which when dissolved in water give acids. However, some oxides are neutral also. For example:
(a) Carbon gives carbon dioxide which is acidic oxide and give carbonic acid on reaction with water.
C(s) + O2(g) → CO2(g)
CO2(g) + H2O(l) → H2CO3(l) (Carbonic acid)
(b) Similarly, sulphur gives sulphur trioxide which on reaction with water gives sulphuric acid.
2S(s) + 3O2(g) → 2SO3(g)
SO3(g) + H2O(l) → H2SO4(aq) (Sulphuric acid)
(c) Oxides like CO, N2O etc., are neutral. They do not give acidic test with moist litmus paper.
(iii) Reaction with water: Non-metals do not react w1th water or steam to evolve hydrogen gas. This is because non-metals cannot donate electrons to reduce hydrogen ions of water to produce hydrogen gas.
(iv) Reaction with acids: Like metals, non-metals do not react with acids. A non-metal being an electron acceptor does not supply electron to H IOIIS and thus cannot react with acids.
(v) Reaction with hydrogen: Non-metals, in general, combine wlth hydrogen to form hydrides (binary) which are covalent compounds,
For example, Water (H2O); hydrogen sulphide (H2S), ammonia (NH3) and hydrochloric acid (HCl) are well known hydrides.
O2(g) + 2H2(g) →2H2O(g)
S(s) + H2(g) → H2S(g)
N2(g) + 3H2(g) → 2NH3(g)
Cl2(g) + H2(g) → 2HCl(g)
(vi) Reaction with chlorine: Non-metals react with chlorine to form their respective covalent chlorides which are non-electrolytes. These are formed by sharing of electrons and thus are covalent compounds (chlorides).
For example, carbon tetrachloride (CCl4), and phosphorus pentachloride (PCl5).
(vii) Reaction with salt solutions: A more reactive non-metal displaces a less reactive non-metal from its salt solution. For example, when chlorine is passed through a solution of potassium bromide, then potassium chloride and bromine are formed.
2KBr(aq) + Cl2(g) → 2KCl(aq) + Br2(l)
(viii) Non-metals are oxidising agents: Non-metals act as oxidising agents as these can accept electron/s from other substances and are themselves reduced.
Cl2 + 2e → 2Cl
Chlorine is thus reduced to chloride.
Remember : Though non-metals are small in number, their presence is vital for the existence of life. Non-metals form the major constituents of air, ocean and earth. Main constituents of air are oxygen and nitrogen. Chlorine occurs in the ocean as chlorides. Earth crust contains non-metals like oxygen, silicon, phosphorus and sulphur in order of their abundance.
| Know This :
Carbon does not behave as a non-metal on account of the following properties:
Reasons to believe that carbon is a non-metal :
|
How do metals and non-metals react? :
Metals are electropositive elements and have a tendency to lose electron/s and acquire a positive charge. While non-metals are electronegative elements and tend to gain electron/s and acquire a negative charge. These positive and negative charges thus attain the electronic configuration of nearest noble gas and then are held by strong electrostatic forces of attraction and salts are formed.
Example:
Sodium atom (II) has electronic configuration: 2, 8, 1. It has one electron in its outermost shell (M). It loses one electron and the outermost shell (now L) has a stable octet. Since nucleus has 11 protons and the number of electrons remained is only 10, there is a net positive charge and we get sodium cation:
Na (2, 8, 1) → Na+ (2, 8) + e
Chlorine atom (17) has electronic configuration: 2, 8, 7. It has seven electrons in its outermost shell (M). It requires one more electron to complete its M-shell. It is possible if chlorine atom reacts with sodium which has a tendency to lose one electron. After gaining one electron, the number of electrons in chlorine atom becomes one more than the number of protons. Thus it gets a net negative charge.
Cl (2,8,7) \(\underrightarrow{+e}\) Cl ( 2,8,8)
Na+ and Cl− ions attract each other by strong electrostatic forces of attraction and exist as sodium chloride (NaCl).
Click on below links to get PDF from store
PDF : Class 10th-Science-Chapter-3-Metals and Non-Metals-Text Book
PDF : Class 10th-Science-Chapter-3-Metals and Non-Metals-Notes
PDF : Class 10th-Science-Chapter-3-Metals and Non-Metals-Solution
Main Page : NCERT-Class-10-Science – All chapters notes, solutions, videos, test, pdf.
Previous Chapter : Chapter-2- Acids, Bases and Salts – Online Notes
Next Chapter : Chapter-4- Carbon and its Compounds – Online Notes
We reply to valid query.