Acids, Bases and Salts
Class-10-CBSE-NCERT-Science-Chapter-2
Notes Part-1
|
Topics to be learn : Part-1
|
Understanding the chemical properties of acids and bases :
Physical characteristics of acids and bases :
Acids :
- are sour in taste.
- change the colour of blue litmus to red
Bases :
- are bitter in taste.
- change the colour of red litmus to blue
Acid-base indicators : Indicators are chemical substances which give different colour in acidic or basic solutions.
Examples :
- Methyl orange - gives pink colour with acid solution and yellow colour with base solution.
- Phenolphthalein (synthetic indicator) - is colourless in acid solution while it turns into pink colour in base solution..
- Litmus solution - turns red in acid solution and blue in base solution.
- Bromothymol blue - is blue in base solution and is yellow in acid solution.
- Natural indicators from plant: (i) Litmus, (ii) Vanilla extract.
| Indicators | ||||
| Sample Solutions | Red litmus solution | Blue litmus solution | Phenolphthalein solution | Methyl orange solution |
| HCl
H2SO4 HNO3 CH3COOH NaOH Ca(OH)2 KOH Mg(OH)2 NH4OH |
no change
no change no change no change turns blue turns blue turns blue turns blue turns blue |
turns red
turns red turns red turns red no change no change no change no change no change |
colourless
colourless colourless colourless turns pink turns pink turns pink turns pink turns pink |
pink
pink pink pink yellow yellow yellow yellow yellow |
Antacids : Antacids are mild alkalies. These are used for getting relief from acidity and indigestion and sometimes, even headache. When taken orally, it reacts with hydrochloric acid present in the stomach and reduces its strength by consuming some of it.
- Example : Milk of Magnesia is an antacid.
Olfactory indicators : Olfactory indicators are substances which have different odour in acid and base solutions.
Examples of Olfactory indicators and characteristics :
- Onion has a characteristic smell. When a base (like NaOH) is added to a cloth strip treated with onion extract—then the smell is destroyed. An acid solution (HCl) does not destroy the smell of onion.
- Vanilla extract has a characteristic pleasant smell. If a basic solution like sodium hydroxide solution is added to vanilla extract, then we cannot detect the pleasant smell of vanilla. An acidic solution does not affect the smell of vanilla.
- Similarly odour of clove oil is not affected in acidic solutions.
Reaction of dilute acids with metals and metal oxides :
(i) Metals and Acids:
Many metals react with dilute acids to give metal salts and hydrogen. The rate of effervescence of hydrogen depends on the reactivity of the metal.Higher rate of effervescence means higher reactivity of the given metal.
- The reactions of metals with hydrochloric acid and dilute sulphuric acid are similar. With dilute hydrochloric acid (HCl) they give metal chlorides and hydrogen; with dilute sulphuric acid (H2SO4), they give metal sulphates and hydrogen.
- Nitric acid is an oxidising agent, so it reacts differently.
2Na + 2HCl → 2NaCl + H2 (Sodium + hydrochloric acid → Sodium chloride)
Mg + 2HCl → MgCl2 + H2 (Magnesium + hydrochloric acid → Magnesium chloride)
Zn + 2HCl → ZnCl2 + H2 (Zinc + hydrochloric acid → Zinc chloride)
(ii) Metal Oxides and Acids: Metal oxides dissolve in dilute acids to give salt and water
Na2O + 2HCl → 2NaCl + H2O (Sodium Oxide + hydrochloric acid → Sodium chloride + water)
MgO + 2HCl → MgCl2 + H2O (Magnesium Oxide + hydrochloric acid → Magnesium chloride + water)
CuO + 2HCl → CuCl2 + H2O (Copper Oxide + hydrochloric acid → Copper chloride + water)
Neutralization reaction : When the effect of a base is nullified by an acid and vice versa, it is called neutralization reaction. In general, a neutralization reaction is written as:
Base + Acid → Salt + Water
Examples:
(i) Aqueous solution of base, NaOH is neutralized by aqueous hydrochloric acid
NaOH(aq) + HCl(aq) → NaCl(aq) + H2O
(ii) Aqueous solution of sulphuric acid is neutralized by aqueous solution of sodium hydroxide.
H2SO4(aq) + 2NaOH(aq) ——> Na2SO4(aq) + 2H2O
Non-metallic oxides : Non-metallic oxides in water form acidic solution.
Example : Non-metallic oxide, carbon dioxide in water forms carbonic acid. We can prove it because aqueous solution of carbon dioxide turns blue litmus red. Further aqueous solution of carbon dioxide is neutralized by a base, calcium hydroxide [Ca(OH)2], to form salt and water.
CO2 + Ca(OH)2 + H2O → CaCO3 + 2 H2O
Q. Explain with the help of an activity that metal oxides are basic and non-metal oxides are acidic in nature (CBSE 2014, 2015)
Ans : Take magnesium ribbon and sulphur powder. On burning, magnesium ribbon will give magnesium oxide (MgO) and sulphur gives sulphur dioxide (SO2) and sulphur trioxide (SO3). Mg is a metal and sulphur is a non-metal.
Now add MgO in water. The solution obtained will turn red litmus blue. It shows that metal oxides are basic in nature.
Similarly, pass SO2 or SO3 in water. Add blue litmus paper to the solution obtained. It will turn red. This shows non-metal oxides are acidic in nature.
Q. Explain why sour substances like lemon or tamarind juice are effective in cleaning the vessels (CBSE 2011, 2014)
Ans : Lemon or tamarind juice is acidic in nature and reacts with oxidised copper (compounds) to dissolve it into soluble salt and water. Thus, copper vessels are cleaned.
Various kinds of oxides and their properties :
Oxides are of three types:
- Acidic oxides.
- Basic or metallic oxides.
- Amphoteric oxides.
Acidic oxides: These oxides on treatment with water form acids, e.g., CO2, SO2, etc.
CO2 + H2O → H2CO3
SO2 + H2O → H2SO3
Thus acidic oxides turn blue litmus red.
Basic or metallic oxides: The oxides which on treatment with water form alkalies are known as basic oxides. Metallic oxides are generally basic oxides. Such oxides turn red litmus to blue, e.g., Na2O, MgO, etc.
Na2O + H2O → 2NaOH
MgO + 2 H2O → Mg(OH)2
Amphoteric oxides: The oxides which show the properties of both acidic and basic oxides are known as amphoteric oxides, e.g., Al2O3, SiO2 etc.
Al2O3 + 6HCl → 2AlCl3 + 3H2O
Al2O3 + 2NaOH → 2NaAlO2 + H2O
Characteristics of acids:
(i) They are sour in taste.
(ii) They turn blue litmus to red.
(iii) Acids react with most metals to liberate H2 gas which burns with a pop sound,
2HCl + Mg → MgCl2 + H2 ↑
H2SO4 + Zn → ZnS04 + H2 ↑
2HCl + 2Na → 2NaCl + H2 ↑
(iv) Acids react with bases to form salt and water. This is called neutralisation
HCI + NaOH → NaC1 + H2O
H2SO4 + Ca(OH)2 → CaSO4 + 2 H2O
(v) Acids react with basic oxides to form salt and water.
CaO + 2HCl → CaCl2 + H2O
Na2O + H2SO4 → Na2SO4 + H2O
(vi) Acids react with carbonates to form salt, Water and carbon dioxide.
CaCO3 + 2HCl → CaCl2 + H2O + CO2 ↑
MgCO3 + H2SO4 → MgSO4 + H2O + CO2 ↑
Acids react with limestone to liberate CO2 gas which turns lime water milky.
Q. Why are acids not stored in metal containers? Containers/vessels made from which material are safe to store acids?
- Metals like sodium, magnesium and calcium react vigorously with mineral acids and give hydrogen.
- Aluminium, zinc, and iron react less vigorously with mineral acids.
- However, some metals like silver and gold do not react with acids.
- Some metals like sodium and calcium react with sulphuric acid violently and are unsafe.
- So mineral acids react with metals and produce corrosion on the surface of metal container.
- Therefore, acids are not stored in metal containers.
- Sulphuric acid, hydrochloric acid and nitric acid are mineral acids. Vessels made from glass or ceramic are considered safe for storing mineral acids,
Bases : Bases are the hydroxide of metals, which give hydroxide ion after dissociation in aqueous solution.
Characteristics of bases
(i) They are bitter in taste.
(ii) They change red litmus to blue.
(iii) They react with acids to form salt and water.
(iv) Common bases are soluble in water and are known as alkalies.
(v) When an alkali reacts with metal, it produces salt and hydrogen.
- Example : When sodium hydroxide reacts with zinc metal, it gives sodium zincate and hydrogen. NaOH + Zn → Na2ZnO2 + H2
(vi) They react with non-metallic oxides to give salt and water.
- Non-metallic oxides are acidic in nature and these react with bases giving neutralization reaction, i.e. salt and water.
- For example when carbon dioxide (non-metallic oxide) reacts with sodium hydroxide, salt and water are formed.
CO2 + 2NaOH → Na2CO3 + H2O
(vii) Bases react with only very active metals to liberate H2 gas which burns with a pop sound.
(viii) Bases react with oils to form soapy solution.
What do all acids and all bases have in common? :
Acids and bases :
According to Arrhenius,
- An acid is a substance which dissolves in water to give hydronium ions, H3O+.
- A base is a substance which dissolves in water to give hydroxyl ions, OH−.
Know this :
|
Q. Give reasons:
(i) Solution of sulphuric acid conducts electricity whereas alcohol does not.
(ii) Dry ammonia gas has no action on litmus paper but a solution of ammonia in water turns red litmus paper to blue. (CBSE 2012, 2015)
(i) Solution of sulphuric acid has charged ions H+ and SO4−2 which help in conducting electricity whereas alcohol does not give any ions in water.
(ii) Dry ammonia has no H+ or OH− ions whereas ammonia in water gives OH− ions which turns red litmus to blue.
Q. Give reason : Acid must be added to water and not vice versa during dilution. (CBSE 2012, 2015)
When an acid is mixed with water, there is evolution of a large amount of heat.
Therefore, acid is slowly added to water. If on the other hand, water is added to acid, it might spill on your body and clothes due to explosion and evolution of sudden and large amount of heat. Therefore it is always desirable to add acid slowly to water, keeping the solution continuously stirred, while preparing dilute solutions of acids, particularly nitric acid and sulphuric acid.
Bases and alkalies : .
- Bases generate hydroxide (OH−) ions in water.
For example,
NaOH(s) \(\overset{H_2O}{\rightarrow}\) Na+(aq) + OH−(aq)
KOH(s) \(\overset{H_2O}{\rightarrow}\) K+(aq) + OH−(aq)
Mg(OH)2(s) \(\overset{H_2O}{\rightarrow}\) Mg2+(aq) + 2 OH−(aq)
- Thus, NaOH, KOH and Mg(OH)2 are bases.
- Alkalies are hydroxides of metals which dissolve in water. Thus, NaOH, KOH, Ca(OH)2 etc. are alkalies also.
- But all bases are not alkalies. For example, aluminium hydroxide [Al(OH)3] is a base but it is not fully soluble in water and so is not an alkali.
Effect on the concentration of hydronium ions (H3O+) when a solution of an acid is diluted :
When a given amount of an acid is added to water, there are a fixed number of hydronium ions per unit volume of the solution. On dilution the number of hydronium ions per unit volume decreases and concentration of hydronium ion decreases.
Q. How is the the concentration of hydroxide ions (OH−) affected when excess base is dissolved in a solution of sodium hydroxide ?
Ans. The concentration of hydroxide ions will increase when excess base is dissolved in a solution of sodium hydroxide because the amount of hydroxide ions per unit volume increases. This happens only when base added dissolves in water. If the base is not soluble in water, the concentration of hydroxide ions remains constant.
How strong are acid or base solutions? :
Universal indicator : An indicator which passes through a series of colour changes over a wide range of H3O+ ion concentration is called universal indicator.
- It is a mixture of several indicators.
- It is used to get approximate idea of pH of the solution.
pH scale : pH scale is the scale for measuring hydrogen ion concentration in a solution.
- pH scale is calibrate from 0 (zero)-—very acidic to 14—very alkaline.
- Values less than 7 represent an acidic solution.
- Values more than 7 represent an alkaline solution.
- Neutral solution has a pH equal to 7.
| pH value | Nature of solution |
| 0-2
2-4 4-7 7 7-10 10-12 12-14 |
Strongly acidic
Moderately acidic Weakly acidic Neutral Weakly basic Moderately basic Strongly basic |
pH : pH is a number which indicates the acidic or basic nature of a solution. Higher the hydronium ion concentration, lower is the pH value. pH is defined as the negative logarithms of hydronium ion concentration.
pH = −ln [H3O+]
pH of neutral water : The pH of neutral water is 7. It means that the concentration of hydronium ions, H3O+ and hydroxyl ions, OH− is equal, i.e., 10−7 moles per litre.
Strength of an acid or a base :
- The strength of an acid or a base depends on the number of H+ ions or OH− ions produced respectively by its given amount.
- If we take one molar concentration (1 mole acid dissolved in 1 litre of solution) of hydrochloric acid and acetic acid, then the acid which gives rise to more of H+ ions is a stronger acid and the one that gives less H+ ions is a weaker acid.
- In this case, it is found that hydrochloric acid is a strong acid.
- Similarly one can find whether it is a strong base or a weak base. (Here number of OH− ions is counted.)
pH decreases with increase of acidic nature (increase of H+ ions)- PH increas
with increase of basic nature (increase of OH− ion concentration).
Examples :
- Strong acids : HCl, H2SO4
- Weak adds : H2CO3, CH3COOH
- Strgng base : KOH
- Weak base : NH4OH
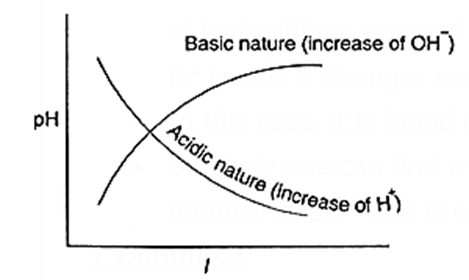
Note : If the pH is less than 7, suppose 3, it does not mean the solution has only H+ ions. It only means that the concentration of H+ ions is greater than those of OH− ions and the solution is acidic. In aqueous solutions, water generates both H+ and OH− ions in equal amounts, but very small concentrations. When acid is added to water, concentration of H+ ions far exceeds those of OH− ions. (Similarly concentration of OH− far exceeds H+ ions when a base is added to water.)
The colours produced by universal indicator at various pH values :
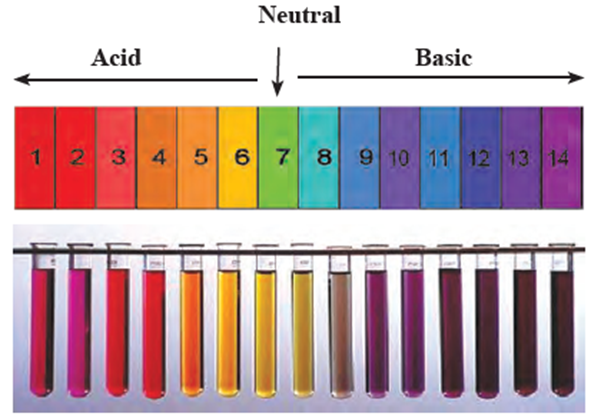
pH value and colour :
| pH
0 1 2 3 4 |
Colour
Dark Red Red Orange red Orange |
pH
5 6 7 8 9 |
Colour
Orange yellow Greenish yellow Green Greenish blue Blue |
pH
10 11 12 13 14 |
Colour
Navy blue Purple Dark purple Violet Violet |
Thus, if on putting the drop of a solution on the universal indicator paper, the paper turns orange, the pH will be about 4 and the solution will be acidic. If the solution turns universal indicator purple, then the pH will be about 11 and the solution will be moderately basic.
Examples : pH value of solutions, colour of pH paper and nature of solutions.
| Solution | Colour of pH paper | Approx pH value | Nature of substance |
| Saliva (before meal) | Light green | 7.4 | Basic |
| Saliva (after meal) | Pale yellow | 5.8 | Acidic |
| Lemon juice | Pink red | 2.5 | Acidic |
| Colourless aerated drink | Pale yellow | 6 | Acidic |
| Carrot juice | Light orange | 4 | Acidic |
| Coffee | Orange yellow | 5 | Acidic |
| Tomato juice | Dark orange | 4.1 | Acidic |
| Tap water | Green | 7 | Neutral |
| 1M NaOH | Dark blue, violet | 13-14 | Basic |
| 1M HCl | Red | 1 | Acidic |
Q. What is acid rain? How does it affect our aquatic life?
Ans. When the pH of rain water is less than 5.6, it is called acid rain. When acid rain
flows into the rivers, it lowers the pH of the river water. Since our body works within a narrow pH range close to 7 (7.0—7.8), the survival of aquatic life in river waters mixed with rain water becomes difficult.
Importance of pH in everyday life :
pH in our digestive system: Our stomach produces hydrochloric acid. This dilute hydrochloric acid helps in digesting our food without harming the stomach. It regulates metabolism activity in the body.
- Sometimes, excess of acid is produced in the stomach for various reasons such as overeating. The excess acid in the stomach causes indigestion which produces pain and irritation.
- In order to cure indigestion and get rid of pain, we can take bases called antacids. Being basic in nature, antacids react with excess acid in the stomach and neutralize it.
- The two common antacids used for curing indigestion due to acidity are magnesium hydroxide (Milk of Magnesia) and sodium hydrogen carbonate.
pH change as the cause of tooth decay:
- When we eat food containing sugar, then the bacteria present in our mouth break down the sugar to form acids such as lactic acid. Thus, acid is formed in the mouth after a sugary food has been eaten.
- This acid lowers the pH in the mouth making it acidic. Tooth decay starts when the pH of acid formed in the mouth falls below 5.5.
- Tooth enamel made up of calcium hydroxyapatite (a cryptalline form of calcium phosphate) is the hardest substance in our body. It does not dissolve in water but when the acid becomes strong enough to attack the enamel of our teeth and corrode it. This sets in tooth decay.
- The best way to prevent tooth decay is to clean the mouth thoroughly after eating food by rinsing it with lots of clean water.
- Many tooth pastes contain bases to neutralise the mouth acid. The pH of tooth paste is about 8.0. Therefore, using the tooth paste, which generally basic, for cleaning the tooth can neutralise the excess acid in mouth and prevent tooth decay.
Soil pH and plant growth:
- Most of the plants grow best when the pH of the soil is close to 7.
- If the soil is too acidic or too basic the plants grow badly or do not grow at all.
- The soil may be acidic or basic naturally. The soil pH is also affected by the use of chemical fertilisers in the fields.
- Most often the soil in the fields is too acidic. If the soil is too acidic, (having low pH), then it is treated with materials like quick lime (calcium oxide) or slaked lime (calcium hydroxide) or chalk (calcium carbonate).
- If the soil is too alkaline then its basicity is reduced by adding decaying organic matter which are acidic.
pH range and survival of animals:
- Our body works well within a narrow pH range of 7.0 to 7.8. If due to some reason, this pH range gets disturbed in the body of a person, then many ailments can occur. The aquatic animals (like fish) can survive in river water within a narrow range of pH.
- When the pH of rain water is about 5.6, it is called acid rain. Too much acid rain can lower the pH of river water to such an extent (and make it so acidic) that the survival of aquatic animals becomes difficult. The high acidity of river water can even kill the aquatic animals (like fish).
- Acids are also present on other planets. For example, the atmosphere of planet Venus is made up of thick white and yellowish clouds of sulphuric acid. Hence, life cannot exist on the planet Venus.
Click on below links to get PDF from store
PDF : Class 10th-Science-Chapter-2-Acids, Bases and Salts-Text Book
PDF : Class 10th-Science-Chapter-2-Acids, Bases and Salts-Notes
PDF : Class 10th-Science-Chapter-2-Acids, Bases and Salts-Solution
Main Page : NCERT-Class-10-Science – All chapters notes, solutions, videos, test, pdf.
Previous Chapter : Chapter-1- Chemical Reactions and Equations – Online Notes
Next Chapter : Chapter-3- Metals and Non-Metals – Online Notes
We reply to valid query.