Elements of Group 13, 14 and 15
Maharashtra State Board-Class-11-Science-Chemistry-Chapter -9
Notes-Part-2
Topics to be Learn : Part-2
|
Allotropy :
Allotropy : When a solid element exists in different crystalline forms with different physical properties (such as colour, density, melting point etc.) the phenomenon is called allotropy. Individual forms are called allotropes.
- In the p-block elements other than carbon, elements such as boron, silicon, bismuth also exhibit allotropy.
Allotropes of Carbon :
The important allotropes of carbon are (i) Diamond (ii) Graphite (iii) Fullerenes (iv) Carbon nanotubes (v) Graphene.
(i) Diamond :
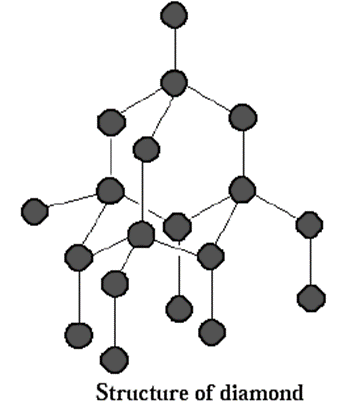
Structure of diamond :
- Diamond has three dimensional structure.
- In this, carbon atom undergoes sp3-hybridisation.
- Each carbon atom is bonded tetrahedrally to four other carbon atoms which occupy the four corners of the tetrahedron, by overlapping of sp3-hybrid orbitals. The C—C bond distance is 154 pm.
- Each of these four carbon atoms are covalently bonded to four more carbon atoms in the Similar fashion and the pattern is repeated in the entire crystal lattice. These tetrahedra are linked together into a three dimensional network to form a giant molecule of diamond.
Properties of diamond :
- Diamond is very hard with high melting point (3873 K) and high density (3.51) (due to its structure).
- It is a non conductor of electricity as there are no free electrons in the crystal structure.
Uses of diamond : Diamond is used
- for cutting glass and in drilling tools.
- for making dies for drawing thin wire from metal.
- for making jewellery.
(ii) Graphite
Graphite is thermodynamically most stable form (allotrope) of carbon.
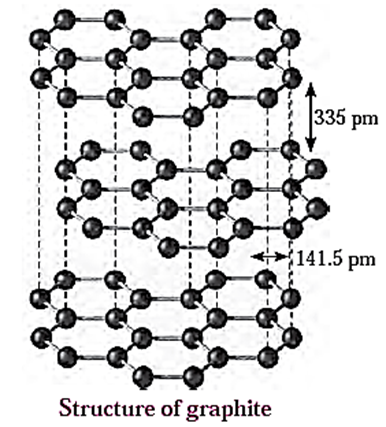
Structure of graphite :
- Planar hexagonal rings of carbon atoms are arranged in layers in a two-dimensional sheet-like structure called graphite.
- As a result of sp2-hybridization, each carbon atom in a hexagonal ring forms three sigma bonds with the other three carbon atoms. Graphite has a C-C bond length of 141.5 pm.
- All carbon atoms share the fourth electron in the unhybrid p-orbital of each carbon atom.
- The distance between the various layers, which is 335 pm, is held by weak van der Waals forces.
- Graphite is very soft and slippery because it cleaves easily between the layers. It is therefore a very effective dry lubricant.
- The electrons in graphite are delocalised over whole sheet. Due to presence of mobile electrons graphite is a good conductor of electricity.
Properties of graphite :
- Graphite is soft and slippery solid.
- It is a good conductor of electricity.
Uses of graphite :
- Graphite is used as a dry lubricant in machines running at high temperature.
- It is also used as electrodes in electrolytic cells and in electrochemical cell like dry cell.
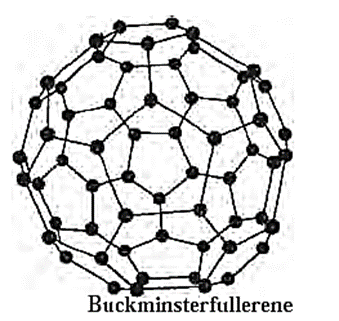
(iii) Fullerenes :
- Fullerene is an allotrope of carbon with definite numbers of carbon atoms.
- Graphite is heated in an electric arc under an inert atmosphere of argon or helium to produce fullerenes. Fullerenes C32, C50, C70, and C84 are only present in trace amounts in the soot that is produced.
- C60, also known as Buckminster fullerene or bucky ball, has a shape similar to a soccer ball. It has fused carbon atom rings that are twenty hexagonal and twelve pentagonal in shape. The distance between the nearby carbon atoms is 143.5 and 138.3 pm.
- Fullerenes are covalent and soluble in organic solvents.
- Fullerene C60 reacts with group 1 metals forming solid such as K3C60 which behaves as a super conductor below 18 K. It carries electric current with zero resistance.
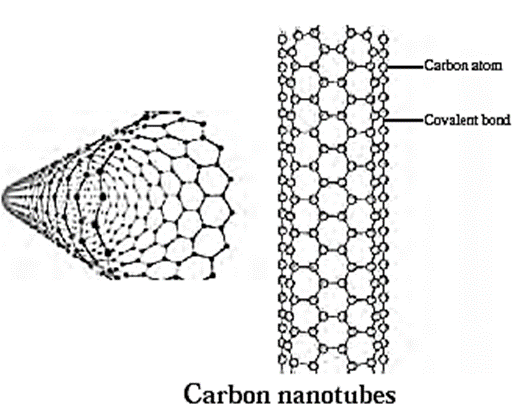
(iv) Carbon nanotubes :
- Carbon nanotubes are cylindrical in shape consisting of rolled up graphite sheets.
- Nanotubes can be single walled (SWNTs) with a diameter less than 1 nm or multi walled (MWNTs) with diameter upto 100 nm. Their length range from several micrometers to milimeters.
- Carbon nanotubes are robust. They can be bent, and when released spring back to the original shape.
- They have high electrical or heat conductivity and highest strength to wait ratio (for any known material).
- Researchers at NASA are combining carbon nanotubes with other materials which can be used to build light weight space craft.
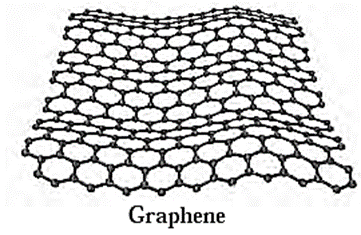
(v) Graphene :
- Isolated layer of graphite is called graphene (Fig.).
- Graphene sheet is a two dimensional solid.
- Graphene has unique electronic properties.
Allotropes of phosphorus :
Phosphorus is found in different allotropic forms, the important ones being white and red phosphorus.
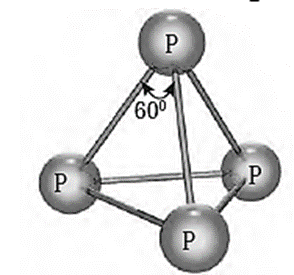
(i) White (yellow) phosphorus :
Structure and properties :
- White (yellow) phosphorus consists of discrete tetrahedral P4 molecules. The P-—P—P bond angle is 60°.
- It is less stable and more reactive because of angular strain in the P4 molecule.
- It is translucent white waxy solid which glows in dark (Chemiluminescence).
- It is insoluble in water but dissolves in boiling NaOH solution.
- It is poisonous.
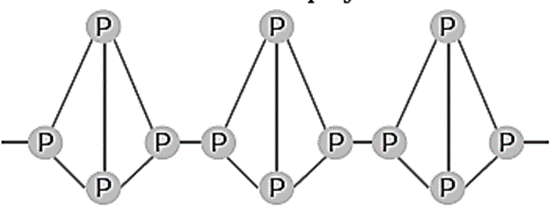
(ii) Red phosphorus :
- Red phosphorus consists of chains of P4 tetrahedra linked together by covalent bonds. Thus it is polymeric in nature.
- It is stable and less reactive.
- It is odourless. It possesses iron grey lustre
- It does not glow in dark.
- It is insoluble in water.
- It is non poisonous.
Remember : Red phosphorus is prepared by heating white phosphorus at 573 K in an inert atmosphere.
Molecular structures of some important compounds of the group 13, 14 and 15 elements :
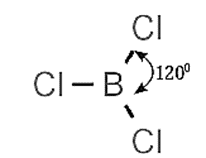
(i) Boron trichloride (BCl3) :
- Bond angle Cl —B — Cl is 120°.
- Boron trichloride is covalent.
- Boron atom undergoes spz-hybridisation with one vacant unhybridised p-orbital.
- Boron in BCl3 has incomplete octet.
- BCl3 molecule is non polar trigonal plannar molecule.
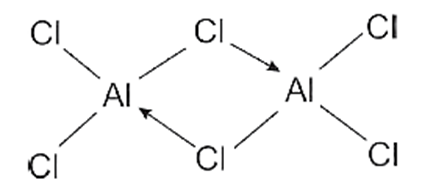
(ii) Aluminium Chloride (AlCl3) :
- Aluminium atom in AlCl3 is sp2-hybridised with one vacant unhybrid p-orbital.
- AlCl3 exists as a dimer, AlCl6.
- Al2Cl6 is formed by the overlap of vacant 3d orbital of Al with lone pairs of electron of Cl.
- AlCl3 is an incomplete octet molecule.
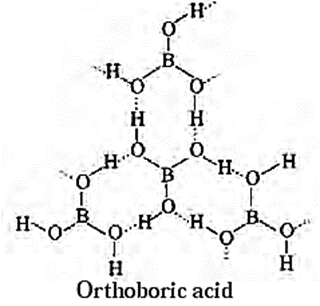
(iii) Orthoboric acid / boric acid (H3BO3) :
- Orthoboric acid has central boron atom bound to three — OH groups.
- The solid orthoboric acid has layered crystal structure.
- The geometry of the molecule is trigonal planar with B(OH)3 units are joined together by hydrogen bonds.
(iv) Diborane (B2H6) :
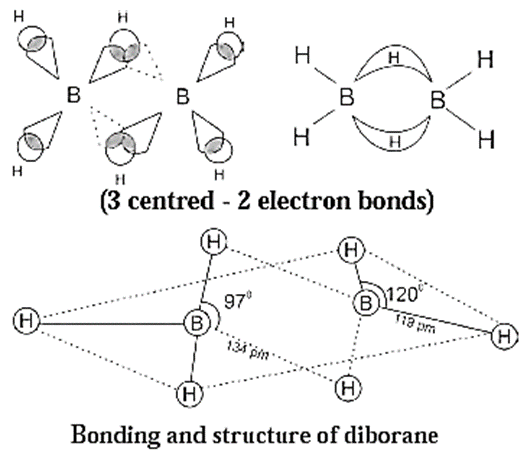
- Boron has electronic configuration, 5B : 1s2 2s2 2p1
- Boron has only three valence electron.
- In diborane each boron atom is sp3
- Three of such hybrid orbitals are half filled, the fourth sp3 hybrid orbital is vacant.
- Two of the sp3-orbitals of each B atom overlap axially with 1s—orbital of two terminal hydrogen atoms forming four terminal B-H, normal covalent sigma bonds. They are called two-centre, two-electron bonds or (2c — 2e) bonds.
- Two boron atoms are linked through two B—Hb—B bridge bonds, each of which is formed by overlapping of sp3 orbitals of two boron atoms and s-orbital of hydrogen atom. The bridge bonds are weak bonds.
- These bonds are called three centre two electron (3c-2e) bonds, since they involve three nuclei and two electrons. Due to their resemblance to banana, they are called banana bonds.
- In diborane molecule, four Ht and two B atoms lie in the same plane while one Hb lies above and another Hb below the plane.
- B2H6 molecule involves 8 bonds and 12 bonding electrons namely 6 electrons from two B atoms and 6 electrons from 6H atoms. Hence B2H6 an electron deficient molecule.
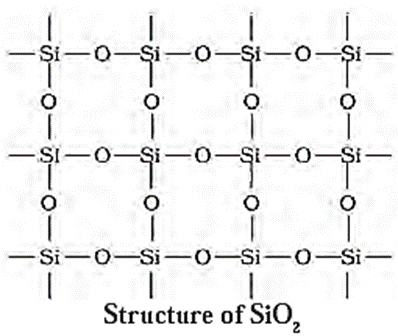
(v) Silicon dioxide (SiO2) :
- Silicon dioxide (SiO2) is commonly named as silica.
- SiO2 is a 3-dimensional network solid.
- Each silicon atom is covalently bonded with four oxygen atoms tetrahedrally and each oxygen atom is covalently bonded to another silicon atom.
- Each corner is shared with another tetrahedron.
- The entire structure is considered as a giant molecule in which eight membered rings are formed with alternate Si and O atoms.
- Quartz, cristobalite and tridymite are the different crystalline forms of silica.
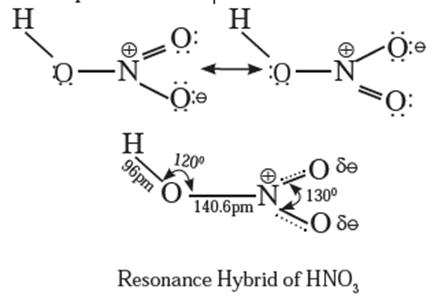
Nitric acid (HNO3) :
- Nitric acid is a strong, oxidising mineral acid.
- The central nitrogen atom is sp2
- HNO3 exhibits resonance phenomenon.
Orthophosphoric acid/phosphoric acid (H3PO4) :
- Phosphorus forms number of oxyacids.
- Orthophosphoric acid is a strong non toxic mineral acid.
- It contains three ionizableacidic hydrogens.
- The central phosphorous atom is tetrahedral.

Chemistry of notable compounds of elements of groups 13, 14 and 15
(i) Borax (Na2B4O7) :
- It is one of the most important boron compound.
- The crystalline borax has formula Na2B4O7.10H2O or Na2[B4O5(OH)4].8H2
Preparation :
- Borax is prepared from the mineral colemanite by boiling it with a solution of sodium carbonate.
Ca2B6O11 (Colemanite) + 2Na2CO3 \(\underrightarrow{Δ}\) Na2B4O7 (Borax) + 2NaBO2 + 2CaCO3 ↓
- On crystallisation it forms Na2B4O7.10H2O (It is a white solid).
Properties :
- Borax is white crystalline solid.
- Borax on hydrolysis, forms a strong base, NaOH and a weak acid, orthoboric acid H3BO3. Due to strong base NaOH, borax solution is alkaline.
Na2B4O7 + 7H2O → 2NaOH + 4H3BO3
- When borax Na2B4O7.10H2O is heated, it loses water molecules and forms a swollen fluffy mass.
Na2B4O7.10H2O \(\underrightarrow{Δ}\) Na2B4O7 + 10H2O
- On further heating, the swollen mass turns into a transparent liquid which solidifies into glass like material known as borax bead.
Na2B4O7 \(\underrightarrow{Δ}\) 2NaBO2 (Sodium metaborate) + B2O3 (boric anhydride)
- Borax bead test is used to detect coloured ions in aqueous solutions.
- When this bead is dipped in a solution of coloured salt [for example CoO (cobalt oxide)] and heated in an oxidising flame of bunsen burner, a blue coloured bead is obtained.
CoO + B2O3 \(\underrightarrow{Δ}\) Co(BO2)2 (blue bead)
- Borax when heated with ethyl alcohol and concentrated H2SO4 acid, give volatile vapours of triethyl borate which burn with green edged flame. This flame test is used to detect borate radical (BO3−3) in the qualitative analysis.
Na2B4O7 + H2SO4 + 5H2O \(\underrightarrow{Δ}\) Na2SO4 + 4H3BO3
H3BO3 + 3C2H5OH → B(OC2H5)3 (Triethyl borate) + 3H2O
Uses of borax :
Borax is used :
- In the manufacture of optical and hard borosilicate glass.
- As a flux for soldering and welding.
- As a mild antiseptic in the preparation of medicinal soaps.
- In qualitative analysis for borax bead test.
- As a brightener in washing powder.
(ii) Silicones :
- Silicones represent organosilicon polymers where {R2SiO} is a repeating unit.
- These are held together by
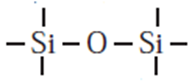 linkage.
linkage. - Silicones have empirical formula R2SiO (R = CH3 or C6H5 group). This is similar to that of ketone (R2CO) and hence the name silicones.
Preparation :
- The starting materials for the manufacture of silicones are alkyl or aryl substituted silicon chlorides of general formula RnSiCl(4_n), where R is an alkyl or aryl group.
- When silicon (Si) and methyl chloride react in the presence of copper catalyst at 573 K, various types of alkyl or methyl substituted chlorosilanes like MeSiCl3, Me2SiCl2, Me3SiCl and Me4Si (tertramethyl silane TMS) are obtained.
- The hydrolysis of dimethyl chlorosilane (CH3)2SiCl2 followed by condensation polymerisation give straight chain polymers called silicones.
- The formation of silicones can be explained by following reactions :
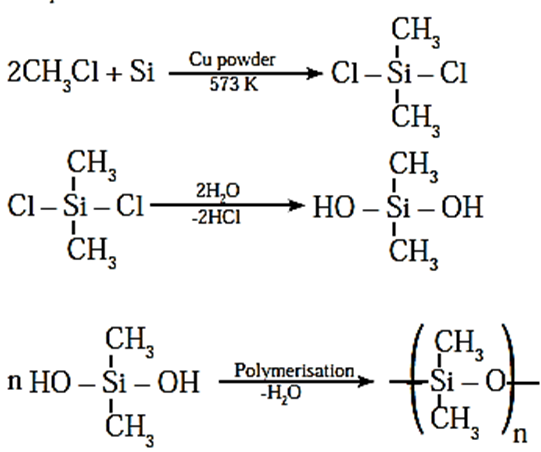
- The chain length of polymer can be controlled by adding (CH3) 3SiCl which blocks the ends of the chain. ((CH3) 3SiOH is used for planar silicones)
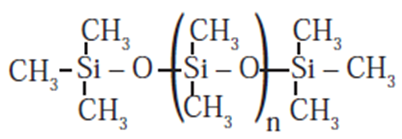
Properties of silicones
- They are water repellant.
- They have high thermal stability.
- They are good electrical insulators.
- They are resistant to oxidation and chemicals.
Uses of silicones
They are used as
- insulating material for electrical appliances
- water proofing of fabrics
- sealant
- high temperature lubricants
- For mixing in paints and enamels to make them resistant to high temperature, sunlight and chemicals.
(iii) Ammonia (NH3) :
Preparation
- Ammonia is present in small quantities in air and soil where it is formed by the decomposition of nitrogeneous organic matter such as urea.
NH2CONH2 (urea) + 2H2O → (NH4)2CO3 ⇌ 2NH3 + H2O + CO2
- Ammonia is prepared on laboratory scale, by decomposition of the ammonium salts with calcium hydroxide or caustic soda.
2NH4Cl + Ca(OH)2 → 2NH3 + CaCl2 + 2H2O
(NH4)2SO4 + 2NaOH → 2NH3 + Na2SO4 + 2H2O
- On the large scale ammonia is prepared by direct combination of dinitrogen and dihydrogen. (Haber’s process)
- In the process, dinitrogen and dihydrogen react directly at high pressure of 200 x 105 Pa (200 atmosphere) and temperature of 700 K.
- The catalyst used is iron oxide with traces of K2O and Al2O3 as promoters. Under these conditions equilibrium is attained rapidly.
N2(g) + 2H2(g) ⇌ 2NH3(g); ΔfHο = −46.1 kJ mol-1
Properties :
Physical properties
- Ammonia is colourless gas with pungent odour.
- It has freezing point of 198.4 K and boiling point of 239.7 K.
- It is highly soluble in water. The concentrated aqueous solution of NH3 is called liquor ammonia.
Remember : Ammonia has higher melting point and boiling point, because in the solid and liquid state NH3 molecules get associated together through hydrogen bonding. Thus some more energy is required to break such intermolecular hydrogen bonds.
Chemical properties
Basic nature :
- The aqueous solution of ammonia is basic in nature due to the formation of OH−
NH3(g) + H2O(l) → NH4+(aq) + OH(aq)
- Ammonia reacts with acids to form ammonium salts.
NH3 + HCl → NH4Cl
2NH3 + H2SO4 → (NH4)2SO4
- Aqueous solution of ammonia precipitates out as hydroxides (or hydrated oxides) of metals from their salt solutions.
ZnSO4(aq) + 2 NH4OH(aq) → Zn(OH)2(s) (White ppt) + (NH4)2SO4(aq)
FeCl3(aq) + 2NH4OH(aq) → Fe2O3.xH2O + NH4Cl(aq)
Complex formation :
- The lone pair of electrons on nitrogen atom of ammonia helps formation of complex compounds with transition metal ions. Hence, it is a good complexing agent. The following reactions show the formation of complexes.
Cu2+(aq) + 4NH3(aq) → [Cu(NH3)4]2+(aq) ..(deep blue)
AgCl(s) + 2NH3(aq) → [Ag(NH3)2]Cl(aq) ..(colourless)
Uses :
Ammonia is used in
- Manufacture of fertilizers such as urea, diammonium phosphate, ammonium nitrate, ammonium sulphate etc.
- Manufacture of some inorganic compounds like nitric acid.
- Refrigerant (liq. ammonia).
- Laboratory reagent in qualitative and quantitative analysis (aq. solution of ammonia)
Remember : Ammonia gives brown ppt with Nessler’s reagent (alkaline solution of K2HgI4).
2KI + HgCl2 → HgI2 + 2KCl
2KI + HgI2 → K2HgI4 (Nesseler’s reagent)
2K2HgI4 + NH3 + 3 KOH → H2N-HgO-HgI (Millon’s base-Brown ppt) +7KI + 2H2O
PDF : Class-11-Chemistry-Chapter-9-Elements of Group 13, 14 and 15- Notes
PDF : Class-11-Chemistry-Chapter-9-Elements of Group 13, 14 and 15-Solution
All 16 Chapters Notes -11-Chemistry-(16 PDF) Rs.132
All 16 Chapters-Solutions-11-Chemistry- (16 PDF) Rs.128
All 16 Chapters-Notes+Solutions-11-Chemistry- (32 PDF) Rs.228
Main Page : – Maharashtra Board Class 11th-Chemistry – All chapters notes, solutions, videos, test, pdf.
Previous Chapter : Chapter-8-Elements of Group 1 and 2 – Online Notes
Next Chapter : Chapter-10-States of Matter – Online Notes