Elements of Group 1 and 2
Maharashtra State Board-Class-11-Science-Chemistry-Chapter -8
Notes-Part-2
Topics to be Learn : Part-2
|
Some important compounds of elements of s-block :
Sodium Carbonate (washing soda) Na2CO3.10H2O
Preparation: Sodium Carbonate is commercially prepared by Solvay process, which takes place in the following stages.
Stage 1: CO2 is passed into a concentrated solution of NaCl which is saturated with NH3.
Crystals of sodium bicarbonate separate as a result of the following reactions.
2NH3(aq) + H2O + CO2(g) → (NH4)2CO3(aq)
(NH4)2CO3(aq) + H2O + CO2 → 2NH4HCO3(aq)
NH4HCO3(aq) + NaCl(aq) → NH4Cl(aq) + NaHCO3(s)
Sodium bicarbonate crystals precipitate out because of its low solubility.
[NaHCO3 is formed as a result of double decomposition reaction in equation (ii)].
Stage 2 : Sodium bicarbonate crystals are heated to obtain sodium carbonate.
2NaHCO3(s) \(\underrightarrow{Δ}\) Na2CO3(s) + H2O(g) + CO2(g)
In this Solvay process the recovery of ammonia is done by treating the solution of NH4Cl obtained with slaked lime, Ca(OH)2. The byproduct of this reaction is calcium chloride.
2NH4Cl(aq) + Ca(OH)2(s) → 2NH3(g) + CaCl2(aq) + H2O(l)
Properties of Sodium Carbonate :
- Sodium carborate (washing soda) is a white crystalline solid having the formula Na2CO3, 10H2O.
- It is highly soluble in water.
- On heating the decahyadrate loses water molecules to form monohydrate.
- On heating above 373 K temperature monohydrate further loses water and changes into white anhydrous powder called soda-ash.
Na2CO3.10H2O(s) \(\underrightarrow{373\,\,K}\) Na2CO3.H2O(s) + 9H2O(g)
Na2CO3.H2O(s) \(\underrightarrow{373\,\,K}\) Na2CO3.(s) + H2O(g)
- Aqueous solution of sodium carbonate is alkaline because of its hydrolysis by the following reaction:
Na2CO3 + H2O → NaHCO3 + NaOH
- The solution becomes alkaline as NaOH is a strong base.
Uses of sodium carbonate :
- Sodium carbonate is used as a cleaning material (as due to its alkaline nature it has emulsifying effect on grease and dirt).
- It is used to make hard water soft (as a water softener), as it precipitates out the soluble calcium and magnesium salts in hard water as carbonates. Thus water becomes soft and gives lather with soap.
For example : Ca(HCO3)2(aq) + Na2CO3(aq) → CaCO3(s) + 2NaHCO3(aq)
- It is used for commercial production of soap and caustic soda.
- It is an important laboratory reagent.
Sodium hydroxide (caustic soda) NaOH
Preparation :
(1) Commercially sodium hydroxide is prepared by the electrolysis of aqueous sodium chloride solution in Castner—Kellner cell, using mercury cathode and graphite anode.
(2) The following reactions take place,
NaCl → Na+ + Cl−
(3) Reaction at cathode :
Na+ + e− \(\underrightarrow{Hg}\) Na—Hg.
Sodium ions get reduced to metallic sodium at cathode. At cathode sodium metal combines with mercury to form amalgam, Na—Hg.
(4) Reaction at anode : Cl− ions are oxidised to form chlorine gas at anode.
Cl− → Cl + e−
Cl +Cl → Cl2
The sodium amalgam when treated with water forms sodium hydroxide and hydrogen gas.
2Na—Hg + 2H2O → 2NaOH + 2Hg + H2.
Properties of sodium hydroxide (NaOH) :
- Sodium hydroxide is a white deliquescent solid with melting point 591 K.
- It is highly soluble in water and gives a strongly alkaline solution.
- The surface of the solution absorbs atmospheric CO2 to form Na2CO3.
Uses of sodium hydroxide :
- Sodium hydroxide is used in the purification of bauxite.
- It is used in the manufacture of soap, paper, artificial silk and many chemical compounds.
- It is used in petroleum refining.
- It is used in textile industries (for mercerizing cotton).
- It is used as an important laboratory reagent.
Calcium Carbonate (CaCO3)
Calcium carbonate is found in nature as chalk, limestone or marble.
Preparation :
(1) From slaked lime : When carbon dioxide is bubbled through slaked lime Ca(OH)2, water insoluble calcium carbonate is formed.
Ca(OH)2(aq) + CO2O(s) → CaCO3(s) + H2O(l)
Controlled addition of CO2 is essential. Excess CO2 transforms precipitate of CaCO3 into water soluble calcium bicarbonate.
(2) From calcium chloride : When solution of calcium chloride is added to a solution of sodium carbonate, calcium carbonate is formed as a precipitate.
CaCl2(aq) + Na2CO3(aq) → CaCO3(s) + 2NaCl(aq)
Physical properties of calcium carbonate :
- Calcium carbonate is soft, light, white powder.
- It is insoluble in water.
Action of heat on calcium carbonate :
On heating to 1200 K, calcium carbonate decomposes into calcium oxide and carbon dioxide.
CaCO3(s) \(\underrightarrow{1200\,\,K}\) CaO(s) + CO2(g)
Action of dilute acids on calcium carbonate :
Calcium carbonate reacts with dilute acids to give corresponding calcium salt and carbon dioxide.
CaCO3 + 2HCl → CaCl2 + CO2 ↑ + H2O
CaCO3 + H2SO4 → CaSO4 + CO2 ↑ + H2O
Uses of calcium carbonate:
- Calcium carbonate in the form of marble is used as building material.
- Calcium carbonate is used in the manufacture of quicklime (CaO) which is the major ingredient of cement.
- A mixture of CaCO3 and MgCO3 is used as flux in the extraction of metals from ores.
- It is required for the manufacture of high quality paper.
- It is an important ingredient in toothpaste, chewing gum, dietary supplements of calcium and filler in cosmetics.
Hydrogen peroxide (H2O2) :
Hydrogen peroxide is a low cost, clean and mild oxidizing agent. A 30% aqueous solution hydrogen peroxide is commercially available.
Preparation :
(i) From hydrated barium peroxide : When hydrated barium peroxide, BaO2.8H2O is acidified with dilute sulphuric acid, hydrogen peroxide is obtained along with a white precipitate of barium sulphate.
BaO2.8H2O(s) + H2SO4(dil) \(\underrightarrow{273\,\,K}\) BaSO4(s) + H2O2(aq) + 8H2O(l)
Insoluble barium sulphate is filtered off to get hydrogen peroxide solution.
(ii) Merk process : Small quantity of sodium peroxide is added to ice cold solution of dilute sulphuric acid, with stirring gives hydrogen peroxide.
Na2O2(aq) + H2SO4(aq) \(\underrightarrow{273\,\,K}\) H2O2(aq) + Na2SO4(aq)
(iii) From electrolytic oxidation of sulphuric acid : A 50% solution of sulphuric acid is subjected to an electrolytic oxidation to form peroxydisulphuric acid at anode.
2HSO4 \(\underrightarrow{electrolysis}\) H2S2O8 + 2e−
Hydrolysis of peroxydisulphuric acid yields hydrogen peroxide.
HO—SO2—O—O—SO2—OH + 2H2O → 2 H2SO4+ H2O2.
[This method can be used to prepare D2O2 in laboratory].
(iv) industrial preparation of hydrogen peroxide :
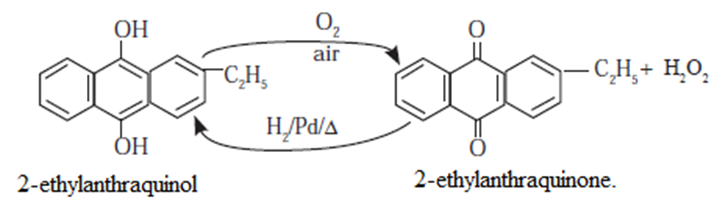
- Industrially hydrogen peroxide is prepared by the air oxidation of 2-Ethylanthraquinol.
- Air is bubbled through a solution of 2-ethylan-thraquinol to get hydrogen peroxide and the oxidised product (2-ethylanthraquinone).
- 2-Ethylanthraquinone is further reduced by H2 gas in presence of Pd catalyst to give back 2-Ethylanthraquinol.
Properties of hydrogen peroxide :
(i) Pure H2O2 is a very pale blue coloured liquid, having b.p. 272.4 K.
(ii) H2O2 is miscible in water and forms a hydrate (H2O2.H2O)
(iii) Strength of aqueous solution of H2O2 is expressed in ‘volume’ units.
- The commercially marketed 30% (by mass) solution of H2O2 has volume strength of 100 volume. It means that 1 mL of 30% solution of H2O2 will give 100 mL oxygen at STP.
(iv) H2O2 acts as a mild oxidising as well as reducing agent.
- Oxidising action of H2O2 in acidic medium
2Fe2+(aq) + 2H+(aq) + H2O2(aq) → 2Fe3+(aq) + 2H2O(l)
- b. Reducing action of H2O2 in acidic medium
2MnO4 + 6H+ + 5H2O2 → 2Mn2+ + 8H2O + 5O2
Uses of hydrogen peroxide :
- Hydrogen peroxide is used as mouthwash, germicide, mild antiseptic, preservative for milk and wine and bleaching agent for soft materials due to its mild oxidising property.
- Hydrogen peroxide, due to its reducing property, is used as an antichlor to remove excess chlorine from fabrics which have been bleached by chlorine.
- Now a days it is also used in environmental chemistry for pollution control, restoration of aerobic condition to sewage water.
Lithium aluminium hydride (LiAlH4) :
Lithium aluminium hydride is commonly abbreviated as LAH. It has chemical formula
LiAlH4.
Prepartion : Lithium hydride is treated with aluminium chloride to give lithium aluminium hydride
4LiH + AlCl3 → LiAlH4 + 3LiCl
Properties: Lithium aluminium hydride is a colourless solid. It reacts violently with water and even atmospheric moisture.
Uses
(i) LAH is a source of hydride and therefore used as reducing agent in organic synthesis.
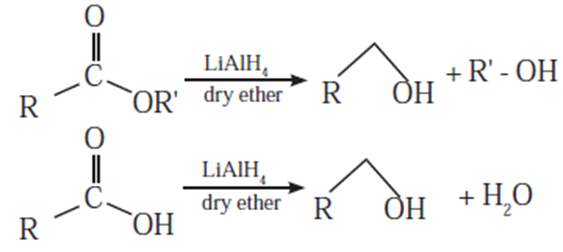
(ii) LAH is useful to prepare PH3(phosphine)
4PCl3 + 3LiAlH4 → 4PH3 + 3AlCl3 + 3LiCl
PDF : Class-11-Chemistry-Chapter-8- Elements of Group 1 and 2- Notes
PDF : Class-11-Chemistry-Chapter-8- Elements of Group 1 and 2-Solution
All 16 Chapters Notes -11-Chemistry-(16 PDF) Rs.132
All 16 Chapters-Solutions-11-Chemistry- (16 PDF) Rs.128
All 16 Chapters-Notes+Solutions-11-Chemistry- (32 PDF) Rs.228
Main Page : – Maharashtra Board Class 11th-Chemistry – All chapters notes, solutions, videos, test, pdf.
Previous Chapter : Chapter-7-Modern Periodic Table – Online Notes
Next Chapter : Chapter-9-Elements of Group 13, 14 and 15 – Online Notes
We reply to valid query.