Basic Principles of Organic Chemistry
Maharashtra State Board-Class-11-Science-Chemistry-Chapter -14
Notes-Part-2
Topics to be Learn : Part-2
|
Isomerism :
The phenomenon of existence of two or more compounds possessing the same molecular formula is known as isomerism.
- Each individual compound is called an isomer.
- Isomerism is mainly of two types : (i) Structural isomerism (ii) Stereoisomerism
Structural isomerism :
When two or more compounds have same molecular formula and different structural formula they are said to be structural isomers of each other. The phenomenon is known as structural isomerism.
Structural isomerism is further classified as
- chain isomerism,
- position isomerism,
- functional group isomerism,
- metamerism
- tautomerism
(i) Chain isomerism : When two or more compounds have the same molecular formula but different parent chain or different carbon skeleton, it is refered to as chain isomerism and such isomers are known as chain isomers.
Example :
- Butane : (CH3 - CH2 - CH2 - CH3)
- 2-methylpropane : [CH3 - CH(CH)3 -CH3]
The above two compounds are chain isomers of each other as they have different skeletons and the same molecular formula C4H10.
(ii) Position isomerism : The phenomenon in which different compounds having the same functional groups at different positions on the parent chain is known as position isomerism.
Example :
- But-2-ene [CH3 - CH = CH - CH3]
- but-1-ene [CH2 = CH - CH2 - CH3]
These are position isomers of each other as they have the same molecular formula C4H8 and the same carbon skeleton but the double bonds are located at different positions.
(iii) Functional group isomerism : The phenomenon in which different compounds with same molecular formula have different functional groups is known as functional group isomerism.
Example :
- Dimethylether (CH3 − O − CH3)
- ethyl alcohol (CH3 − CH2 − OH)
These have same molecular formula C2H6O but the former has ether (− O −) functional group and the latter has alcohol (− OH) functional group.
(iv) Metamerism : Different compounds with the same molecular formula and the
same functional group, but having unequal distribution of carbon atoms on either side of the functional group is refered to as metamerism and the isomers are known as metamers.
Example :
- Ethoxyethane (CH3 − CH2 − O − CH2 − CH3)
- methoxypropane (CH3 − O − CH2 − CH2 − CH3)
These have the same molecular formula C4H10O and the same functional group ether but have different distribution of carbon atoms attached to etheral oxygen. These are metamers of each other.
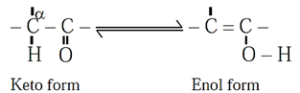
(v) Tautomerism : Same compounds exists as mixture of two or more stucturally distinct molecules which are in rapid equilibrium with each other. This phenomenon is referred to as tautomerism. Such interconverting isomers are called tautomers.
Example :
Keto-enol tautomerism is very common form of tautomerism. It is found in carbonyl compounds. Here a hydrogen atom shifts reversibly from the ∝-carbon of the keto form to oxygen atom of the enol.
Theoretical basis of organic reactions :
Types of cleavage of covalent bond :
A covalent bond can undergo cleavage or fission in two ways
- Homolytic cleavage
- Heterolytic cleavage
(i) Homolytic fission : The symmetrical breaking of covalent bond between two atoms such that each atoms retains one electron of the shared pair forming free radicals is known as homolytic fission.
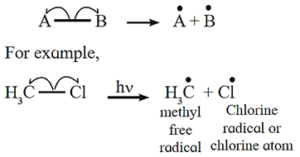
- The movement of a single headed arrow or fish hook
- In this fission each departing species are electrically neutral and contain are unpaired electron is called free radical.
- A free radical is unstable and a reactive species having a tendency to accept an electron for pairing.
- The fission takes place at high temperature or in the presence of UV light or suitable peroxide and in the non polar solvent.
Free radical : A uncharged species with incomplete octet which is electrically neutral and contains an unpaired electron is called free radical. Free radical is highly reactive and short lived.
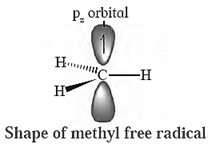
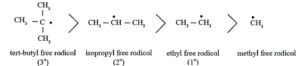
(a) A carbon free radical is sp2 hybridized and reveals planar trigonal geometry.
Alkyl free radicals are classified as primary (10), secondary (20) and tertiary (30).
The observed stability order of alkyl free radicals is :
(ii) Heterolytic fission :
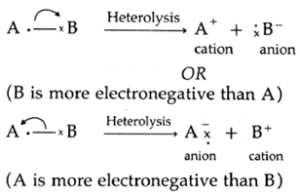
- The unsymmetrical breaking of a covalent bond between two atoms in such a way that one of the departing atoms (more electronegative atom) retains the shared electron pair of the bond, forming charged ions is known as heterolytic fission. This fission can be shown as,
- This fission takes place when two bonding atoms differ largely in electronegativity.
- The more electronegative atom retains electron pair and acquires negative charge (anion) and another atom gets positive charge (cation).
- This fission takes place in a polar solvent since ions are stabilised by solvation.
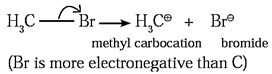
- Organic reactions proceeding by heterolysis are called ionic or heteropolar or polar reactions.
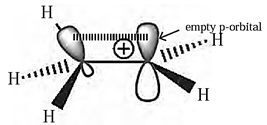
Carbocation :
A carbon atom having sextet of electrons and a positive charge is known as carbocation,
Structure of methyl carbocation :
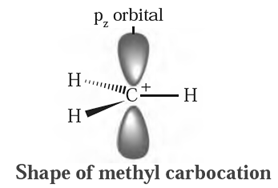
- In methyl carbocation, positively charged carbon atom has six electrons.
- The central carbon atom of carbocation is sp2-hybrid state and has trigonal planar geometry.
- It is bonded to three hydrogen atoms and H-C-H bond angle is 120°.
- The P, atomic orbital is empty and lies perpendicular to the plane containing the three C-H sigma bonds at the carbon.
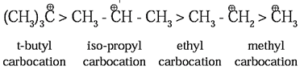
Classification of Carbocations :
Carbocations are classified as primary (10), secondary (20) and tertiary (30). The observed stability of alkyl carbocations follows the order :
Carbanion :
Carbon atom having octet of electrons and a negative charge is known as carbanion.
Heterolytic bond fission generates a species called carbanion in which carbon gets the shared pair of electrons. This happens when a carbon atom is bonded to the electropositive atom.

Structure of methyl carbanion :
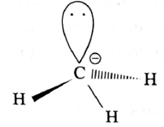
- In methyl carbanion, negatively charged carbon atom has eight electrons.
- The central carbon atom of carbanion is sp3-hybridised and has distorted tetrahedral geometry.
- It is bonded to three hydrogen atoms.
- Carbanion is unstable and reactive species.
- It is favoured in the polar solvents.
Examples of classifications of some bond fissions :

Types of reagent :
There are two types of reagents as follows :
- Electrophiles or Electrophilic centre (H+, NO2+)
- Nucleophiles or Nucleophilic centre (OH—, Cl—)
Electrophiles or Electrophilic centre :
- The species which are electron seeking species i.e. electron deficient species are called electrophiles or electrophilic centre.
- A polyatomic electrophile has an electron deficient atom in it called the electrophilic centre.
- They are positively charged species like H+, NO2+or neutral molecules with incomplete octet of electrons like BF3, AlCl3
- The electrophilic centre of the electrophile AlCl3 is Al which has only 6 valence electrons.
Nucleophiles or Nucleophilic centre :
- The species which are nucleous seeking species i.e. electron rich species are called nucleophiles or nucleophilic centre.
- A polyatomic nucleophile has an electron rich atom in it is called the nucleophilic centre.
- They are negatively charged species OH—, Cl— or neutral species like H2O in which ‘O’ has two lone pairs of electrons.
Example :
The structural formulae of two reagents NH3 and CH3+ .showing all the valence electrons are :
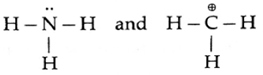
- Thus NH3 contains N with a lone pair of electrons which can be given away to another species. Therefore NH3 is a nucleophile and ‘N’ in it is the nucleophilic centre.
- The CH3+ is a positively charged electron deficient species having a vacant orbital on the carbon. It is an electrophile. The ‘C’ in it is the electrophilic centre.
Electronic effects in organic reaction :
- The displacement of valence electrons resulting in polarization of an organic molecule is called electronic effect.
- The effect that occurs in the ground state is permanent effect.
- This takes place due to influence of certain atom or substituent or certain structural feature present in the molecule.
- Inductive effect and resonance effect are examples of permanent electronic effect.
Electronic displacements in a covalent bond :
- The displacement of electrons in a covalent bond of a molecule of an organic compound takes place either due to the presence of a substituent group, under the influence of an atom or due to the presence of a suitable attacking reagent.
- Electron displacement may be temporary or permanent and sometimes it may lead to the fission of a covalent bond. The electron displacement due to a substituent group or due to the influence of an atom cause permanent polarization of a covalent bond developing a polar character.
Inductive effect :
- When an organic molecule has a polar covalent bond in its structure, polarity is induced in adjacent carbon-carbon single bonds too. This is called inductive effect.
- The more electronegative atom or the group which pulls the bonding electrons is said to have negative inductive effect (—I) and the one which is less electronegative repels the bonding electrons is said to have positive inductive effect (+1). The effect is transmitted along a chain of covalent bonds, due to the mobility of electrons through single bonds.
- Example : In methyl chloride, chlorine is more electronegative than carbon atom. Hence, chlorine pulls the bonding pair of electrons towards itself. Thus, the chlorine atom acquires a fractional negative charge, while the carbon atom acquires a fractional positive charge.
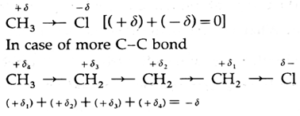
- This mobility or effect is passed on to the subsequent bonds and decreases rapidly with the ‘increase in the number of single bonds, (+δ1 > +δ2 ...).
- The effect becomes insignificant after four C-C bonds.
- The electron withdrawing atoms or groups of atoms having, —I effect are highly electronegative. For example, F, Cl, Br, L.
- The electron repelling atoms or groups of atoms with + I effect are electropositive. For example, metals, alkyl groups, CH3—CH2—CH3
- Inductive effect helps to understand reaction mechanisms.
Resonance structures :
Structure of benzene : The cyclic structure of benzene consists of three alternating C—C single bonds and three C=C double bonds states two distinct bond length of 154 pm and 133 pm respectively.
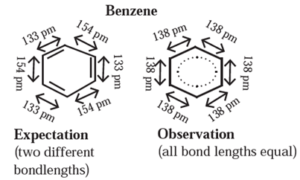
Experimental measurements show that benzene has a regular hexagonal shape and all the six carbon bonds, have the same bond length of 138 pm which is intermediate between C—C single bond and C=C double bond. This means that all the six carbon carbon bonds in benzene are equivalent.
Conjugated system of π bonds : When Lewis structure of a compound has two or more multiple bonds alternating with single bonds, it is called a conjugated system of π bonds.
Resonance theory :
- The π electrons in conjugated system of π bonds are not localized to a particular π
- Compound having a conjugated system of π bonds (or similar other systems) two or more Lewis structures are indicated by showing movement of π electrons (that is delocalization of π electrons) using curved arrows.
- The Lewis structures are linked by double headed arrow and are called resonance structures or contributing structures or canonical structures of the species. Thus, two resonance structures can be drawn for benzene by delocalizing or shifting the π electrons :
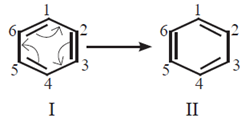
- The positions of the carbon atoms in the conjugated system of = bonds remains the same, but the positions of π electrons are different in different resonance structure. For example, the resonance structure I of benzene has a single bond between C1 and C2 while in the resonance structure I there is a double bond between C1 and C2
- Any resonance structure is hypothetical and by itself does not represent any real molecule and can explain all the properties of the compound.
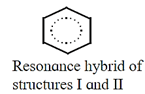
- The real molecule however, has character of all the resonance structures those that can be written. The real or actual molecule is the resonance hybrid of all the resonance structures.
- Example : An actual benezene molecule is the resonance hybrid of structures I and II and exhibits character of both these structures. It can be represented as a dotted circle inscribed in a regular hexagon. Thus, each carbon carbon bond in benzene has single as well as double bond character and the ring has a regular hexagonal shape.
- Hypothetical energy of an individual resonance structure is calculated using bond energy values. The energy of actual molecule is, however, lower than that of any one of the resonance structures. It means that, resonance hybrid is more stable than any of the resonance structures. The difference in the actual energy and the lowest calculated energy of a resonance structure is called resonance stabilization energy or resonance energy. Thus, resonance leads to stabilization of the actual molecule.
Rules to be followed for writing resonance structure :
- Resonance structures can be written only when all the atoms involved in the π conjugated system lie in the same place.
- All the resonance structures must have the same number of unpaired electrons.
- Resonance structures in accordance to their energy or stability contribute to the resonance hybrid. Resonance structure (having low energy) are stable and contribute largely to resonance hybrid and are therefore, important.
Resonance Effect :
The polarity produced in the molecule by the interaction between conjugated π bonds (or that between π bond and p-orbital on attached atom) is called the resonance effect or mesomeric effect. The effect is transmitted through a chain of conjugated π bonds.
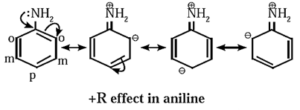
(i) Positive resonance effect or electron donating/releasing resonance effect (+ R effect) :
- If the substituent group has a lone pair of electrons to donate to the attached π bond or conjugated system of π bonds, the effect is called +R effect.
- The groups having lone pair of electrons show +R effect are —OH, —OR, —O— —NHR, —halogen, etc.
- The +R effect increases electron density at certain positions in a molecule. The +R effect in aniline increases the electron density at ortho and para positions.
The following structures are resonance forms.
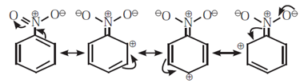
(ii) Negative resonance effect or electron withdrawing resonance effect (—R effect) :
- If the substituent group has a tendency to withdraw electrons from the attached π bond or conjugated system of π bonds towards itself, the effect is called —R effect. For example, groups such as —COOH, —CHO, —CO—, —CN, ~NO,, — COOR, etc. show —R effect.
- The —R effect results in developing a positive polarity at certain positions in a molecule.
- The —R effect in nitrobenzene develops positive polarity at the ortho and para positions.
The following structures are resonance forms.
Electromeric effect :
This is a temporary effect exhibited by multiple bonded groups in the excited state in
the presence of an attacking reagent. When a reagent approaches a multiple bond, the complete transfer of a shared pair of π electrons to one of the atoms joined by a multiple bond, giving a charged separated structure.

This effect is temporary and disappears when the reagent is removed from the reacting system.
Hyperconjugation : Hyperconjugation is a permanent electronic effect explains stability of a carbocation, free radical or alkene. It is delocalization of σ (sigma) electrons of a C—H bond of an alkyl group directly attached to a carbon atom which is part of an unsaturated system or has an empty p-orbital or a p-orbital with an unpaired electron.
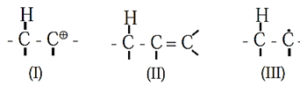
Species stabilized by hyperconjugation
Hyperconjugation in ethyl cation :
- In ethyl cation, CH3—C+H2 positively charged carbon atom is attached to a methyl group.
- Positively charged carbon atom has six electrons due to three bond pairs, namely two C—H bonds and one C—C bond.
- C+ atom in ethyl cation is sp2-hybridised and it has one empty p-orbital available for hyperconjugation.
- The methyl group attatched to C+ rotates around C-C, hence one of its σ C—H bond is always aligned with the plane empty orbital of C+.
- The electrons of σ C-H bond are delocalised into empty p-orbital of C+ and decreases the positive charge and stabilises the ethyl cation.
Hyperconjugation in ethyl carbocation
Thus, hyperconguation in ethyl cation is a σ — π conjugation
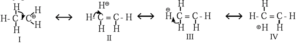
The contributing structures II, III and IV involving delocalization of σ electrons of C-H bond shows no covalent bond between carbon and one of the ∝-hydrogens. Hyperconjugation is, therefore, called ‘no bond resonance’.
Hyperconjugation in propene OR No bond resonance in propene :
- Delocalization of electrons by hyperconjugation is also possible in alkenes. For example, consider hyperconjugation in propene.
- In propene, CH3—CH=CH2, sp3-hybridised carbon atom of methyl group is attached to sp2-hybridisecl carbon atom of the double bond.
- The hyperconjugation involves delocalization of σ C—H bond electrons into p-orbital of a carbon atom of a double bond.
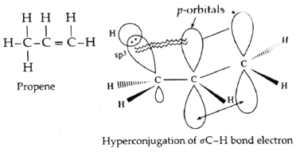
Hyperconjugation structures :

- In the hyperconjugation structures, there is no bond between one of the H atoms and carbon atom attached to a double bond. Hence, this phenomenon is called ‘no bond resonance’. In this, ∝ C—H bond possesses ionic character.
- The effect of hyperconjugation is usually stronger than inductive effect.
PDF : Class-11-Chemistry-Chapter-15-Hydrocarbons-Text Book
PDF : Class-11-Chemistry-Chapter-15-Hydrocarbons- Notes
PDF : Class-11-Chemistry-Chapter-15-Hydrocarbons-Solution
All 16 Chapters Notes -11-Chemistry-(16 PDF) Rs.132
All 16 Chapters-Solutions-11-Chemistry- (16 PDF) Rs.128
All 16 Chapters-Notes+Solutions-11-Chemistry- (32 PDF) Rs.228
Main Page : – Maharashtra Board Class 11th-Chemistry – All chapters notes, solutions, videos, test, pdf.
Previous Chapter : Chapter-13-Nuclear Chemistry and Radioactivity – Online Notes
Next Chapter : Chapter-15-Hydrocarbons – Online Notes