Elements of Group 13, 14 and 15
Maharashtra State Board-Class-11-Science-Chemistry-Chapter -9
Notes-Part-1
|
Topics to be Learn : Part-1
|
Introduction :
p-Block elements : The elements having atoms in which the last electron (differentiating electron) enters the outermost p-orbital, are called p-block elements.
- Position of p-block elements in the periodic table : The p—block elements are placed at the extreme right of the periodic table, after the transition elements. They belong to the groups 13 to 18.
- The p-block elements show greater variation in the properties than 's' block,
The p-block consists of following elements :
(i) Group 13 – Boron Family :
- Elements : boron (5B), aluminium (13Al), gallium (31Ga), indium (49In) and thallium (81Tl)
(ii) Group 14 – Carbon Family :
- Elements : carbon (6C), silicon (14Si), germanium (32Ge), tin (50Sn) and lead (82Pb)
(iii) Group 15 – Nitrogen Family :
- Elements : nitrogen (7N), phosphorous (15P), arsenic (33As), antimony (51Sb) and bismuth (83Bi)
(iv) Group 16 – Chalcogens, i.e., oxygen family :
- Elements : oxygen, sulphur, selenium, tellurium, polonium
(v) Group 17 – Halogens, i.e., fluorine family:
- Elements : fluorine, chlorine, bromine, iodine, astatine.
(vi) Group 18 – Noble gases, i.e., helium family:
- Elements : helium, neon, argon, krypton, xenon, radon.
Electronic configuration of elements of groups 13, 14 and 15 :
The general outer electronic configuration of,
- Group 13 elements is ns2 np1,
- Group 14 elements is ns2np2
- Group 15 elements is ns2np3.
Condensed electronic configuration of elements of groups 13, 14 and 15 :
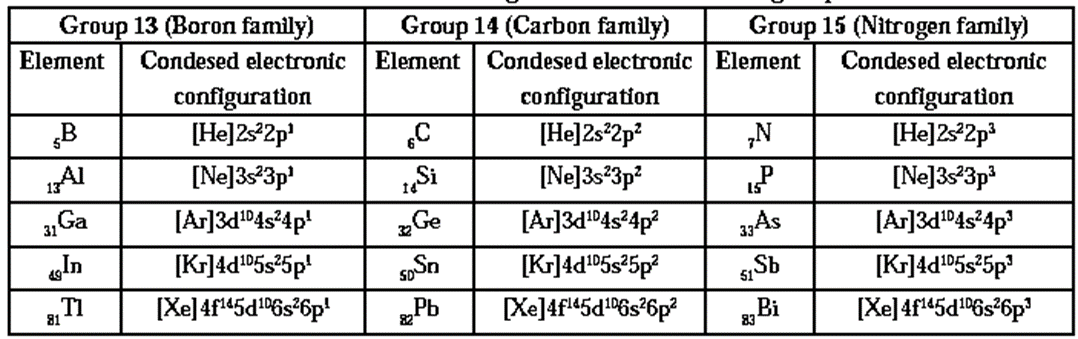
Classification of the elements of groups 13, 14 and 15 into metals, metalloids and nonmetals.
- Elements of group 13 : Boron is a metalloid (glossy hard solid like metals and poor electrical conductor like nonmetals). Other elements Al, Ga, In and Tl are reactive metals.
- Elements of group 14 : Carbon is a nonmetal. Silicon and germanium are metalloids (hard and lustrious like metal and brittle like nonmetals). Tin and lead are corrosion resistant, moderately reactive metals.
- Elements of group 15 : Gaseous nitrogen and brittle phosphorus are nonmetals. Arsenic and antimony are metalloids. Bismuth is a moderately reactive metal.
Trends in atomic and physical properties of elements of groups 13, 14 and 15 :
(i) Group 13 elements :
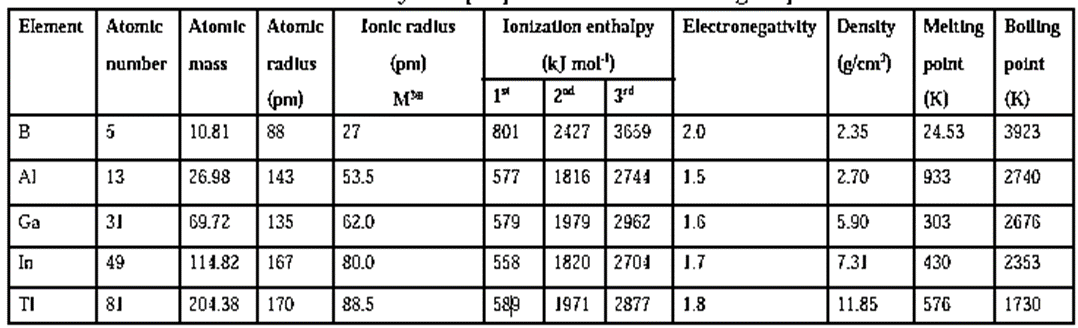
Trend in atomic radii :
- Atomic radius shows irregular trends in the elements of group 13.
- It increases from boron to aluminium.
- Atomic radius of gallium is less than that of aluminium.
- It increases from gallium to thallium
Trend in electron affinity :
- Electron affinity of group 13 decreases down the group from A1 to Tl.
- As the atomic size increases down the group, nuclear attraction on the added electron decreases due to shielding effect and hence electron affinity decreases.
- However, due to its small atomic size B has lower electron affinity than Al.
Trends in ionization enthalpy :
- Ionization enthalpy shows irregular trends in group 13 elements :
- It decreases from B to Al as expected.
- There is marginal difference in ionization enthalpy from Al to Tl.
- Ionization enthalpy increases slightly for Ga but decreases from Ga to In.
- The last element Tl, has higher ionization enthalpy (IE1) due to poor shielding effect of 10-d-electrons and 14-f electrons on outer electrons.
Trends in electronegativity :
- Electronegativity decreases from B to Al.
- There is marginal increase in electronegativity further down the group from Al to Tl due to discrepancies in their atomic size. (refer below table)
Physical properties of elements of group 13 :
| Text Book :
Q. Atomic numbers of the group 13 elements are in the order B < Al < Ga < In < Tl. Arrange these elements in increasing order of ionic radii of M3+.
Ans : The given elements are in an increasing order of atomic number. As we go down the group 13, their general outer electronic configuration is ns2np1. M3+ is formed by removal of three electrons from the outermost shell 'n'. In the M3+ the 'n-1' shell becomes the outermost. Size of the added 'n-1' shell increases down the group. Therefore the ionic radii of M3+ also increase down the group as follows : B3+ < Al3+ < Ga3+ < In3+ < Tl3+
Q. Why the atomic radius of Gallium is less than that of aluminium ?
Ans : Atomic radius increases down the group due to added new shell. 'Al' does not have ‘d’ electrons. As we go from Al down to 'Ga' the nuclear charge increase by 18 units. Out of the 18 electrons added, 10 electrons are in the inner 3d subshell. 'd' Electrons offer poor shielding effect. Therefore, the effects of attraction due to increased nuclear charge is experienced prominently by the outer electrons of 'Ga' and thus its atomic radius becomes smaller than that of 'Al'.
|
(ii) Group 14 elements :
Trend in atomic radii :
- Atomic radii of the elements in group 14 regularly increase down the group from C to Pb due to addition of new valence shell for the each succeeding element.
- However, the increase is comparatively less after silicon due to poor shielding by inner d and f-electrons in the atoms.
Trends in ionization enthalpy :
- Ionisation enthalpy of carbon is very high (1086 kJmol-1) due to small atomic size and high electronegativity.
- It decreases sharply for silicon (786 kJmol-1) as there is an appreciable increase in atomic size and low electronegativity of silicon atom compared to carbon atom.
- Hence, there is phenomenal decrease in ionisation enthalpy from carbon to silicon.
- Even if atomic radius of 82Pb is higher than 50Sn, due to poor shielding effect of inner d and f orbitals, ionisation enthalpy of Pb is more than that of Sn.
Trends in electronegativity :
- Carbon is the most electronegative element among the group 14 elements. The electronegativity decreases from C to Pb.
- However due to presence of d and f electrons after silicon, the electronegativity is nearly same (1.8) for Si to Sn.
- It is slightly more for the last element, lead Pb (1.9).
Some atomic and physical properties of group 14 elements :
| Text Book :
Q. The values of the first ionization enthalpy of Al, Si and P are 577, 786 and 1012 kJmol-1 respectively. Explain the observed trend.
Ans : The trend shows increasing first ionization enthalpy from Al to Si to P. Al, Si and P belong to 13 period in the periodic table. They have same valence shell. Due to the increased nuclear charge electrons in the valence shell are more tightly held by the nucleus as we go from Al to Si to P. Therefore more energy is required to remove an electron from its outermost shell.
|
(ii) Group 15 elements :
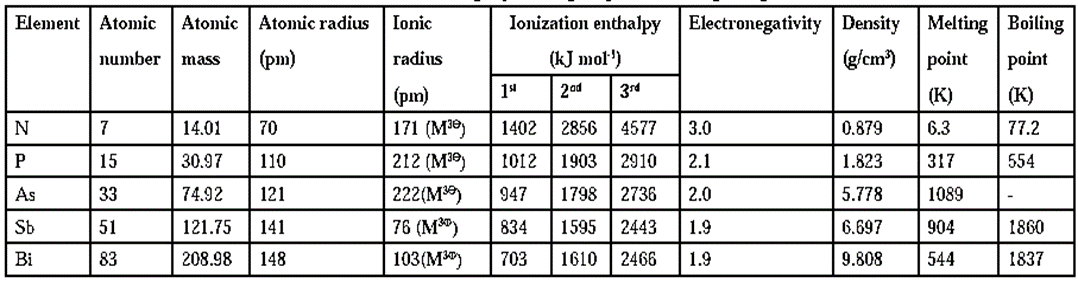
Trend in atomic radii :
- In the group 15 elements, as the atomic number increases down the group, atomic (and ionic) radii increase from Nitrogen to Bismuth.
- There is a considerable increase in atomic radii from N to P but from As to Bi the increase is small.
Trends in ionization enthalpy :
- Due to gradual increase in atomic size of the elements of group 15, down the group ionisation enthalpy decreases from N to Bi.
- Ionisation enthalpy of group 15 elements is much higher than group 14 elements in the same period. This is due to extra stability of half filled p-orbitals and relatively small size of elements.
- The 1st, 2nd and 3rd ionisation enthalpies of these elements are in the order ΔH1 < ΔH2 < ΔH3.
Trends in electronegativity :
- Down the group, as atomic number increases, electronegativity decreases.
- The decreasing order of electronegativity is, N > P > As < Sb = Bi.
Some atomic and physical properties of group 15 elements :
Chemical properties of the elements of the groups 13,14 and 15 :
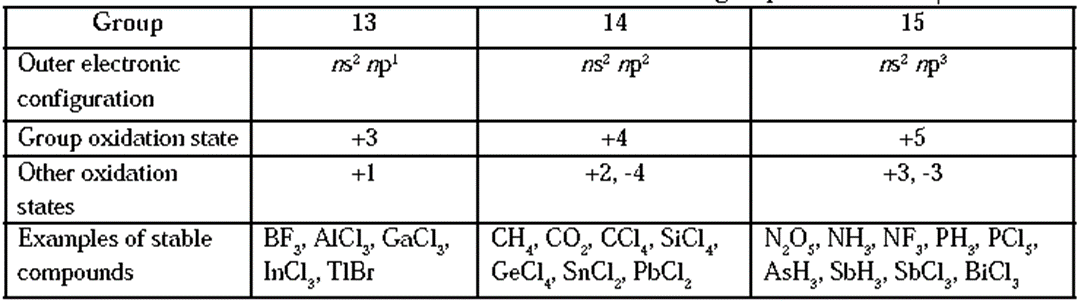
Oxidation state :
- The elements of groups 13, 14 and 15 exhibit various oxidation states.
- The highest oxidation state exhibited by p-block elements is equal to total number of valence electrons (sum of s-electrons and p—electrons). This is called group oxidation state.
- Besides, the elements of groups 13, 14 and 15 exhibit other oxidation states which are lower than the group oxidation state by 2 units. The lower oxidation states become more stable as we go down the group.
Oxidation states of the elements of groups 13, 14 and 15 :
Inert pair effect : In case of heavier elements, the s-electrons in the valence shell remain paired and do not participate in the chemical bonding. This effect is called inert pair effect.
This effect arises due to :
- poor shielding of ns2-electrons by inner d-and f—electrons.
- when the energy required to unpair the paired s electrons is more than the energy evolved in the bond formation in the compound, ns2—electrons remain inert.
Due to this effect many heavier elements show more stable lower oxidation states but the lighter elements of the same group show stable higher oxidation states. For example in group-13, the elements B, Al show 3 + state while Ga, In and Tl show more stable 1 + oxidation state.
Similarly in group-14, the elements C, Si show 4 + state while Sn, Pb show more stable 2 + state along with 4 + .
Remember : The increased stability of the oxidation state lowered by 2 units than the group oxidation state in heavier p-block elements is due to inert pair effect. In these elements, the two selectrons are involved less readily in chemical reactions. This is because the s-electrons of valence shell in p-block elements experience poor shielding than valence p- electron, due to ten inner d-electrons.
Pattern of variation in the oxidation states of boron (B) to Thallium (Tl), (group 13 elements) :
- The elements of group 13 from B to Tl have general valence shell electronic configuration, ns2np1.
- First two elements of group 13 namely, B and Al have stable 3 + oxidation state.
- Stability of 3 + oxidation state starts decreasing and that of lower oxidation state, 1 + starts increasing from Ga to Tl due to inert pair effect.
- Next two elements, Ga and In are normally trivalent but also have 1 + oxidation state in some compounds.
- Thallium has very stable 1 + oxidation state (thallous) than 3 + (thallic) state. Hence Tl (III) salts act as good oxidising agents.
Pattern of variation in the oxidation states of C to Pb in group 14. (group 14 elements) :
- The elements of group 14 from C to Pb have general valence shell electronic configuration, ns2np2
- All the elements show common oxidation state 4 +.
- However as we go down from C to Pb, the stability of higher oxidation state (4 +) decreases while that of the lower oxidation state (2+) increases. This is due to inert pair effect.
- The stability of 2 + state increases in the order of Ge < Sn < Pb.
- Hence, C and Si show only 4 + state while Ge and Sn form 4 + as well as 2 + state. Pb forms very stable 2 + oxidation state, hence, the salts of unstable Pb in 4 + state act as an oxidizing agent.
| Text book :
Q. Why Tl+1 ion is more stable than Tl+3 ?
Ans : Tl is a heavy element which belongs to group 13 of the p-block. The common oxidation state for this group is 3. In p-block, the lower oxidation state is more stable for heavier elements due to inert pair effect. Therefore, Tl+1 ion is more stable than Tl+3 ion.
|
Bonding in compounds of group 13, 14 and 15 elements :
- Because of their small atomic radii and high ionisation enthalpy values, the lighter elements of groups 13, 14, and 15 form covalent bonds.
- Because of the increase in atomic radii and decrease in ionisation enthalpy down the group, the heavier elements in the group tend to form ionic bonds.
- Each group's first member is in the second period and does not have any vacant d-orbitals. They are unable to expand their octet. However, the following elements have empty d-orbitals. As a result, they can expand their octet and form a wide range of compounds.
Reactivity towards air/oxygen
(i) Group 13 elements : Elements of group 13 on heating with air or oxygen produce oxide of type E2O3 (where E = element) and Nitrides
4E(s) + 3O2(g) \(\underrightarrow{Δ}\) 2E2O3(s)
2E(s) + N2(g) \(\underrightarrow{Δ}\) 2EN(s)
(ii) Group 14 elements : The elements of group 14 on heating in air or oxygen form oxide of the type EO and EO2 in accordance with the stable oxidation state and availability of oxygen.
E(s) + O2(g) \(\underrightarrow{Δ}\) EO
E(s) + O2(g) \(\underrightarrow{Δ}\) EO2
(iii) Group 15 elements : The elements of group 15 on heating in air or oxygen forms two types of oxide E2O3 and E2O5.
P4 + 3O2 \(\underrightarrow{Δ}\) P4O6
P4 + 5O2 \(\underrightarrow{Δ}\) P4O10
As4 + 3O2 \(\underrightarrow{Δ}\) As4O6
2Bi + 3O2 \(\underrightarrow{Δ}\) Bi2O3
Increase in metallic character down all these groups 13, 14 and 15 reflects their oxides which gradually vary from acidic through amphoteric to basic.
Nature of stable oxides of groups 13, 14 and 15 elements :
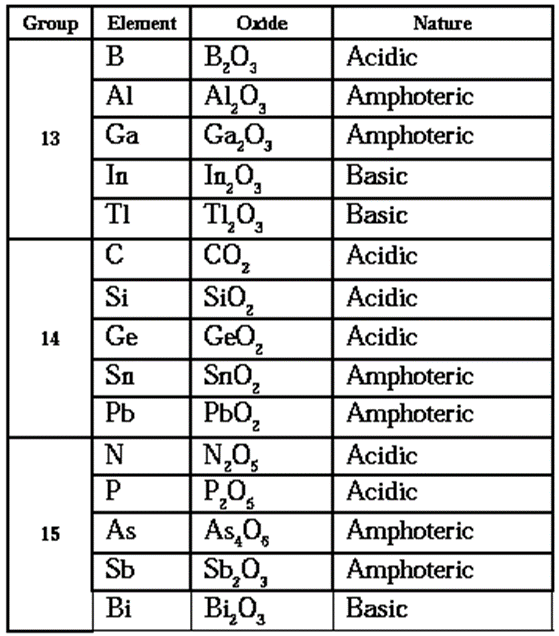
Reaction with water :
- Most of the elements of groups 13,14 and 15 are unaffected by water.
- Aluminium reacts with water on heating and forms hydroxide while tin reacts with steam to form oxide.
2Al(s) + 6H2O (l) \(\underrightarrow{Δ}\) 2Al(OH)3(s) + 3H2(g)
Sn(s) + 2H2O (g) \(\underrightarrow{Δ}\) SnO2(s) + 2H2(g)
- Lead is unaffected by water, due to formation of a protective film of oxide.
Reaction with halogens
Reaction of elements of group 13 with halogens :
- All the elements of group 13 except Tl react directly with halogens to form trihalides (EX3)
2E(s) + 3X2(g) → 2EX3(s) …(E = element, X = halogen)
2Al(s) + 3Cl2(g) → 2AlCl3(s)
- Thallium forms monohalide
2Tl + X2 → 2TlX (X = halogen).
2Tl + Cl2 → 2TlCl (Thallium chloride )
Reaction of elements of group 14 with halogens :
- All the elements of group 14 react directly with halogens to form tetrahalicles. (EX4) (E = element) (X = halogen)
Si + 2Cl2 → SiCl4
- Ge and Pb form dihalides too (GeCl2; PbCl2).
- Stability of dihalides increases down the group due to inert pair effect.
- The ionic character of halides increases clown the group.
Reaction of elements of group 15 with halogens :
- Elements of group 15 react with halogens to form two series of halides. EX3 and EX5
P4 + 6Cl2 → 4PCl3
- The pentahalides (EX5) are more covalent than trihalides (EX3) due to vacant d orbitals of the valence shell available for bonding. P4 + 10Cl2 → 4PCl5. Nitrogen does not form pentahalide.
Remember :
- The pentahalides possess more covalent character due to availability of vacant d orbitals of the valence shell for bonding.
- Nitrogen being second period element (7N : 1s2 2s2 2p3), does not have d orbitals in its valence shell, and nitrogen can have only 3 + oxidation state and not 5 + oxidation state therefore, does not form pentahalides).
- NF3 is the only stable halide of nitrogen
- Trihalides of the group 15 elements are predominantly covalent except BiF3.
| Text Book
Q. GeCl4 is more stable than GeCl2 while PbCl2 is more stable than PbCl4. Explain.
Ans : Ge and Pb are the 4th and 5th period elements down the group 14. The group oxidation state of group 14 is 4 and the stability of other oxidation state, lower by 2 units, increases down the group due to inert pair effect. The stability of the oxidation state 2 is more in Pb than in Ge.
Q. Nitrogen does not form NCl5 or NF5 but phosphorous can. Explain.
Ans : Phosphorous and other members of the group can make use of d-orbitals in their bonding and thus compounds MX3, as well MX5 are formed Nitrogen can not form NCl5 or NF5 since it is void of d-orbitals in its second shell.
|
Catenation : The property of self linking of atoms of an element by covalent bonds to form chains and rings is called catenation.
- The catenation tendency of an element depends upon the strength of the bond formed.
- Among the group 14 elements, carbon shows the maximum tendency for catenation because of the stronger C - C bond.
- The order of catenation of group 14 elements is C >> Si > Ge = Sn. Pb does not show catenation.
PDF : Class-11-Chemistry-Chapter-9-Elements of Group 13, 14 and 15- Notes
PDF : Class-11-Chemistry-Chapter-9-Elements of Group 13, 14 and 15-Solution
All 16 Chapters Notes -11-Chemistry-(16 PDF) Rs.132
All 16 Chapters-Solutions-11-Chemistry- (16 PDF) Rs.128
All 16 Chapters-Notes+Solutions-11-Chemistry- (32 PDF) Rs.228
Main Page : – Maharashtra Board Class 11th-Chemistry – All chapters notes, solutions, videos, test, pdf.
Previous Chapter : Chapter-8-Elements of Group 1 and 2 – Online Notes
Next Chapter : Chapter-10-States of Matter – Online Notes