Chapter-5-Chemical Bonding
Maharashtra State Board-Class-11-Science-Chemistry-Chapter -5
Notes Part-1
|
Topics to be Learn : Part-1
|
Introduction :
The force of attraction which holds the two atoms or ions together in a compound is called a chemical bond.
- There are two ways of formation of chemical bonds (i) by loss and gain of electrons (ii) by sharing a pair of electrons between the two atoms.
- In either process of formation of chemical bond each atom attains a stable noble gas electronic configuration.
- The electrons present in the outermost shell of an atom are involved in the formation of a chemical bond.
Kossel and Lewis approach to chemical bonding :
Electronic theory of valency was put forward by Kossel and Lewis.
According to this theory :
- During bond formation, only the electrons in the outermost shell (called valence electrons) of an atom are involved.
- Chemical inertness and extraordinary stability of noble gases (inert gases) are due to the presence of 8 electrons except He with 2 electrons in their valence shell.
- All the atoms tend to acquire the stable configuration of the nearest noble gas (inert element) by losing, gaining or sharing electrons with other atom.
- H and Li atoms tend to acquire two electrons like He while other atoms tend to possess 8 electrons in their valence shell.
Octet rule : During the formation of a chemical bond, an atom of a particular element gains, loses or shares electrons with other atoms until it acquires a stable electronic configuration of eight electrons (the octet) in its valence shell as in the nearest noble gas, except H atom which acquires 2 electrons like He.
- Octet rule is based on stability of noble gases due to presence of eight electrons (ns2np6) in the valence shell.
Significance of octet rule :
The octet rule is very useful in
- Explaining the normal valency of elements
- The study of chemical combination of atoms leading to formation of molecules.
However it should be noted that octet rule is not valid for H and Li atoms.
Reasons :
- Hydrogen has atomic number 1 and hence has only one electron in its valence shell. It can acquire electronic configuration of helium by acquiring one more electron.
- Lithium has atomic number 3 and has one electron in its valence shell. Lithium atom can acquire the electronic configuration of helium (the nearest noble gas) by losing one electron.
Thus, hydrogen and lithium atoms attain noble gas configuration of two electrons and not an octet of 8 electrons. Hence octet rule is not valid for H and Li atoms
Ionic bond :
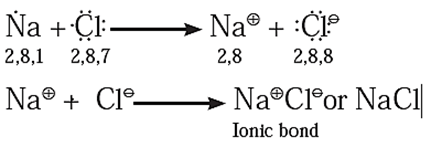
(i) Formation of sodium chloride (NaCl)
The electronic configurations of Sodium and Chlorine are :
Na (Z = 11) 1s22s22p63s1 or 2, 8, 1
Cl (Z = 17) 1s22s22p63s23p5 or 2, 8, 7
- Sodium has one electron in its valence shell. It has a tendency to lose one electron to acquire the configuration of the nearest noble gas Ne (2, 8).
- Chlorine has seven electrons in its valence shell. It has a tendency to gain one electron and thereby acquire the configurationof the nearest nobel gas Ar (2, 8, 8). During the combination of sodium and chlorine atoms, the sodium atom transfers its valence electron to the chlorine atom, sodium atom changes into Na+ ion while the chlorine atom changes into Cl− ion.
- The two ions are held together by strong electrostatic force of attraction.
The formation of ionic bond between Na and Cl can be shown as follows.
Formation of ionic bond in the molecule of calcium chloride :
Calcium atom has electronic configuration,
Ca(=20) 2, 8, 8, 2
It has 2 electrons in the valence shell.
Chlorine atom has electronic configuration,
Cl(= 17) 2, 8, 7
Hence Cl has 7 valence electrons.
- Ca acquires extrastability of noble element Ar by losing 2 electrons and forms a cation with 2 positive charge Ca2+. Cl acquires extrastability of Ar by accepting one electron from Ca and forms an anion Cl−.
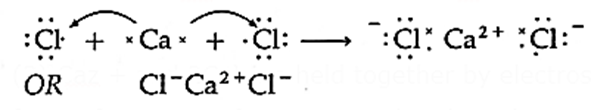
Ca2+ and 2Cl− are held together by electrostatic force of attraction forming ionic bonds and a molecule, CaCl2.
Ionic solids and Lattice Enthalpy :
Ionic solids : Ionic solids are solids which contain cations and anions held together by ionic bonds.
Formation of ionic bonds on the basis of ionisation enthalpy and electron gain enthalpy :
- Ionisation is an endothermic process while electron gain enthalpy can be endothermic or exothermic.
- Elements having low ionisation enthalpy form ionic bond with the elements having a high negative value of electron gain enthalpy.
- Both the above processes take place in the gaseous state.
- All ionic compounds in the solid state have each cation surrounded by specific number of anions and vice versa.
The ionic compounds crystallise from gaseous state to solid state.
M(g) M+ (g) + e−
X(g) + e− → X− (g) electron gain enthalpy
M+ (g) + X−(g) → MX(g) → MX(s)
- The stability of ionic solids depends upon the interactions between the ions and energy released during their formation.
For example, consider the formation of NaCl solid.
Na(g) → Na+(g) + e− ΔiH = 495.8 kJ mol−1
Cl(g) + e−→ Cl−(g) ΔegH = — 348.7 kJ mol−1
Na+(g) + Cl− (g) → NaCl(g) = + 147.7 kJ mol−1
NaCl(g) → NaCl(s) Δ H = — 788 kJ mol−1

| Know This :
CsF is the most ionic compound. Because Cs is the most electropositive while F is the most elctronegative element. The electronegativity difference between them is the largest. Hence ions are easily seperable, the bond is weakest and the compound is least stable ionic compound. |
Lattice Enthalpy : Lattice Enthalpy of an ionic solid is defined as the energy required to completely separate one mole of solid ionic compound into the gaseous components.
Lattice enthalpy of NaCl is -788 kJ mol−1, which means that 788 kJ of energy is required to separate 1 mole of NaCl into one mole of gasesous Na+(g) and Cl−(g) to an infinite distance.
For same anion and different cations :
- Cations having higher charge have large lattice energies than compounds having cations with lower charge. AlCl3 > CaCl2 > NaCl
- As size of cation decrease, lattice energy increases. LiF > NaF > KF.
Covalent bond :
The attractive force which exists due to themutual sharing of electrons between two atoms of similar electronegativity or having small differences in their electronegativity is called a covalent bond. It is represented by a dash (—) between the two bonding atoms.
Example :
(i) Covalent bond formation in H2 molecule :
Hydrogen atom has one electron in its valence shell and its electronic configuration is (H = 1) 1s1.
It needs one more electron to attain the stable configuration of He.
Two hydrogen atoms, at a certain internuclear distance share their valence electrons and they are linked by single covalent bond forming a molecule of H2.
The shared pair of electrons belongs to both the atoms thereby each hydrogen atom completes its doublet.
H· + ·H → H ·· H or H—H
shared electron pair covalent bond
Thus two hydrogen atoms are bonded by a single covalent bond forming H2 molecule.
(ii) Formation of Cl2 :
- The electronic configuration of chlorine is 17Cl. 2, 8, 7. It has 7 electrons in its valence shell.
- The chlorine atom needs one electron to attain stable configuration of argon with a complete octet.
- Two chlorine atoms share a pair of valence electrons.
- Thus each chlorine atom contributes one electron to the shared pair and both the atoms get octet in their valence shell.

- Thus two chlorine atoms are bonded by single covalent bond forming Cl2 molecule.
The dots represent the electrons. Such structures are referred to as Lewis structures.
In H2O and CCl4 the formation of covalent bonds can be represented as,
|
(iii) Formation of Multiple bond :
When two bonding atoms share two or more pairs of electrons, the covalent bond between them is called a multiple bond.
For example, a double bond in O2 and a triple bond in N2. Some other examples of multiple bonds are CO2 and C2H2
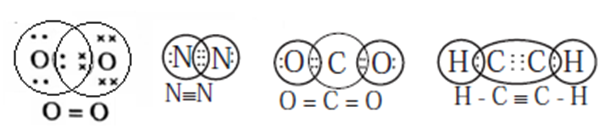
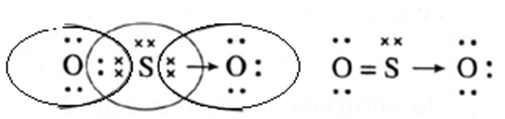
Co-ordinate covalent bond : This bond is formed by sharing a pair of electrons from one atom by two bonding atoms. It is represented by an arrow from a donor atom to an acceptor atom. Example SO2
Distinguish between Ionic bond and Covalent bond :
| Ionic bond | Covalent bond |
| Ionic bond is formed by the transfer of an electron from an electropositive atom to an electronegative atom. | Covalent bond is formed by sharing of a pair of electrons between two bonding atoms. |
| There is electrostatic attraction between the oppositely charged ions (cations and anions) formed in the process. | There is no electrostatic force of attraction between the atoms. |
| In the formation of an ionic bond, one atom donates an electron to the other atom. | In the formation of a covalent bond, each atom contributes one electron. |
| In this, oppositely charged ions are formed. | In this, no ions are formed. |
Lewis structures (Lewis representations of simple molecules) :
Lewis dot structures show a picture of bonding in molecules and ions in terms of the shared pairs of electrons and the octet rule.
Although such a picture does not explain completely the bonding and behaviour of a molecule, it helps to understand the formation and properties of molecule.
Steps to write Lewis dot structures
- Count the total number of valence electrons of each combining atoms in the molecule.
- Write skeletal structure of the molecule to show the atoms and number of valence electrons forming the single bond between the atoms.
- Add remaining electron pairs to complete the octet of each atom.
- If octet is not complete form multiple bonds between the atoms such that octet of each atom is complete.
- In anions add one electron for each negative charge.
- In cations remove or subtract one electron from valence electrons for each positive charge.
- In polyatomic atoms and ions, the least electronegative atom is the central atom for eg. 'S' is the central atom in SO42−, 'N' is the central atom in NO3−.
- After writing the number of electrons as shared pairs forming single bonds, the remaining electron pairs are used either for multiple bonds or remain as lone pairs.
Lewis dot structures and dash structure of some molecules/ions
| Compound | Lewis dot structure | Lewis dash structure |
| CCl4 | 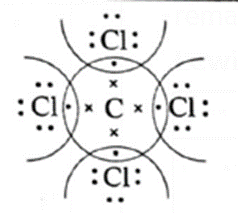 |
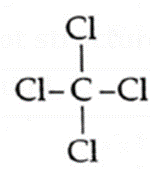 |
| H2O | 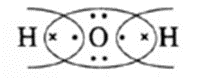 |
|
| CO2 | 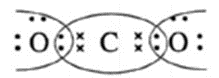 |
|
| HNO3
(H-1)(O-2,6)(N-2,5) |
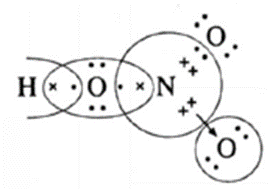 |
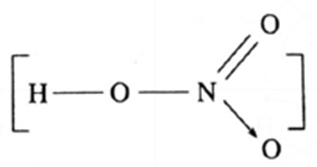 |
| H3PO4
(H-1)(P-2,8,5)(O-2,6) |
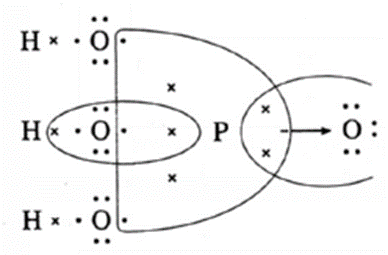 |
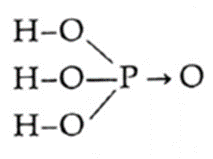 |
| CO32- |  |
 |
Q. To write Lewis structure of nitrite ion, NO2−.
Step I : Count the number of valence electrons of nitrogen and oxygen atoms. There is one additional electron of negative charge.
7N 2,5 and BO 2, 6
Step II : Write the skeletal structure of NO2− by flanking two electronegative oxygen atoms around less electronegative nitrogen atom. Write neagtive charge on one oxygen atom.
Step III : Nitrogen needs 3 electrons and oxygen needs 2 electrons to complete their octet. Indicate corresponding valence electrons.
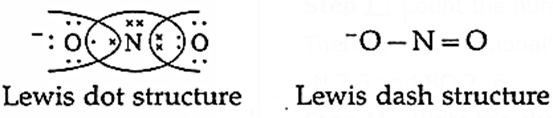
(Circles indicate 8 electrons around each atom.)
Q. Write the Lewis structure of CO molecule.
Step I : Count the number of valence electrons in carbon and oxygen atoms.
6C 2,4 and 8O 2, 6
Step II : Write the skeletal structure of CO with valence electrons.
![]()
Step III : Carbon needs 4 electrons and oxygen needs 2 electrons to complete their octet. Hence rearrange electrons in oxygen atom and write a dative or coordinate covalent bond from oxygen to carbon indicated by an arrow sharing a pair of electrons from oxygen completing an octet of C and O atoms.
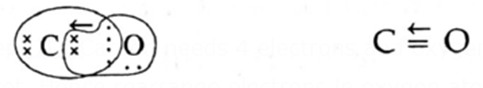
Lewis dot structure Lewis dash structure Hence according to Lewis structure, CO molecule has one double bond and one coordinate or dative bond () but not a triple bond (C ≡ O).
Formal charge : The formal charge of an atom in a polyatomic molecule or an ion is defined as the difference between the number of valence electrons of the atom in an isolated or a free state and the number of electrons assigned to that atom in the Lewis structure.
- Formal charges can help us in assigning bonds when several structures are possible.
- Formal charge is the charge assigned to an atom in a molecule, assuming that all electrons are shared equally between atoms, regardless of their relative electronegativities.
- While determing the best Lewis structure per molecule the structure is chosen such that the formal charge is as close to zero as possible.
- The structure having the lowest formal charge has the lowest energy.
Expression of formal charge (F.C.) :
[Formal charge on an atom in a Lewis structure]
= [Total no. of valence electrons in free atom] − [Total no. of non bonding or lone pairs of electrons] − ½ [Total no. of bonding or shared electrons]
Or FC = VE − NE − ½ BE
Q. Calculate a formal charge on an oxygen atom in H2O.
Ans. Lewis dot structure for H2O molecule is
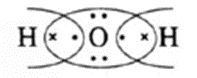
[Formal charge on O atom] = [Total no. of valence electrons in free O atom] − [Total no. of non bonding electrons] − ½ [Total no. shared electrons in bonds]
∴ FC on O atom = 6 − [4} − ½ [4] = 0
Hence, formal charge F.C. on oxygen atom is zero.
Q. Calculate formal charges on oxygen atom in ozone molecule.
Ans. Lewis dot structure for O3 molecule is
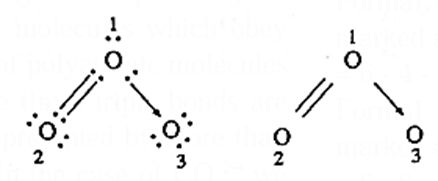
The three oxygen atoms are numbered as 1, 2, 3.
(i) [Formal charge on O1 atom] = [Total no. of valence electrons in free O1 atom] − [Total no. of non bonding electrons] − ½ [Total no. shared electrons in bonds]
FC on oxygen atom O1 atom = 6 − [2] − ½ [6] = +1
(ii) FC on oxygen atom O2 atom = 6 − [4] − ½ [4] = 0
(iii) FC on oxygen atom O3 atom = 6 − [6] − ½ [2] = −1
Q. Assign formal charges on C and two O atoms in CO2 for different structures.
Ans. CO2 has following three structures

(I) Lewis dot structure of structure I of CO2 is,
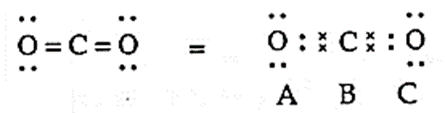
(i) Formal charge on carbon atom (i.e. atom B)
= [Total no. of valence electrons in free C atom] − [Total no. of non bonding electrons] − ½ [Total no. shared electrons in bonds]
FC on C atom = 4 − [0] − ½ [8] = 0
(ii) Formal charge on oxygen atom (i.e. atom A)= 6 − [4] − ½ [4] = 0
(ii) Formal charge on oxygen atom (i.e. atom C) = 6 − [4] − ½ [4] = 0
(II) Lewis dot structure of structure II of CO2 is,

(i) Formal charge on carbon atom (i.e. atom B) = 4 − [0] − ½ [8] = 0
(ii) Formal charge on oxygen atom (i.e. atom A)= 6 − [2] − ½ [6] = +1
(ii) Formal charge on oxygen atom (i.e. atom C) = 6 − [6] − ½ [2] = −1
(II) Lewis dot structure of structure III of CO2 is,

(i) Formal charge on carbon atom (i.e. atom B) = 4 − [0] − ½ [8] = 0
(ii) Formal charge on oxygen atom (i.e. atom A)= 6 − [6] − ½ [2] = −1
(ii) Formal charge on oxygen atom (i.e. atom C) = 6 − [2] − ½ [6] = +1
It is observed that in structure I, the formal charge on all atoms is zero while in structures II and III the formal charge on carbon is zero while oxygen atoms have formal charges — 1 and + 1 respectively.
Limitation of octet rule :
Octet rule fails to explain the following :
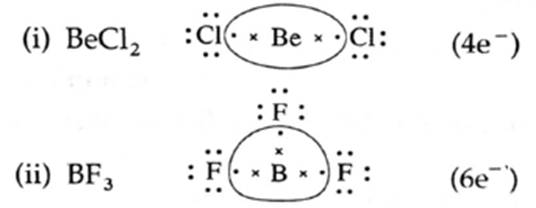
- The stability of incomplete octet molecules, i.e., the molecules with the central atom containing less than eight electrons. For example, BeCl2, BF3.
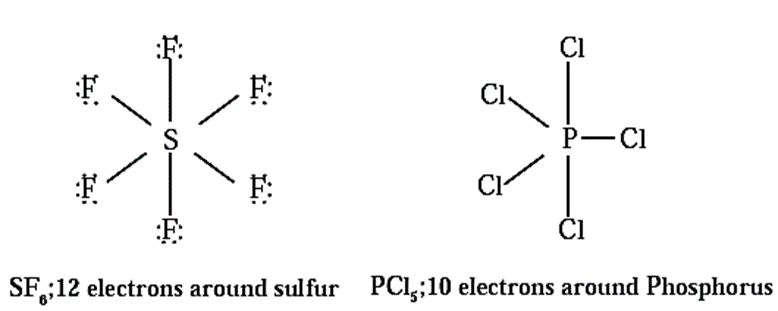
- The stability of expanded octet molecules, i.e., the molecules with the central atom containing more than eight electrons. For example, PCl5, SF6.
- Observed shape and geometry of the molecules.
- Difference in energies and reactivities of different molecules.
(i) Molecules with incomplete octet :
Example : BF3, BeCl2, In these covalent molecules the central atoms B, Be have less than eight electrons in their valence shell but are stable.
- Be in BeCl2 has four electrons while
- B in BF3 has six electrons in the valence shell.
(ii) Molecules with expanded octet :
Some molecules like SF6, PCl5, have more than eight electrons around the central atom.
(iii) Odd electron molecules
- The molecules which do not obey octet rule and have odd number of electrons are called odd electron molecules. For example, NO, NO2.
- Both N and O in NO and NO2 have odd number of electrons.

- They are paramagnetic in nature.
- Their shape and geometry cannot be explained by the octet rule.
- The octet rule fails to explain the difference in reactivities of different molecules.
Q. Why is H2 molecule stable, even though it never satisfies the octet rule ?
- Hydrogen atom has one electron in its valence shell.
- It needs only one more electron to complete a doublet like the nearest inert element helium (He).
- A molecule of hydrogen (H2) is formed by sharing their valence electrons forming a covalent bond.
![]()
- As the shared pair of electrons is common to both the hydrogen atoms they attain a doublet.
- Hence the molecule of H2 is stable even though it does not satisfy octet rule.
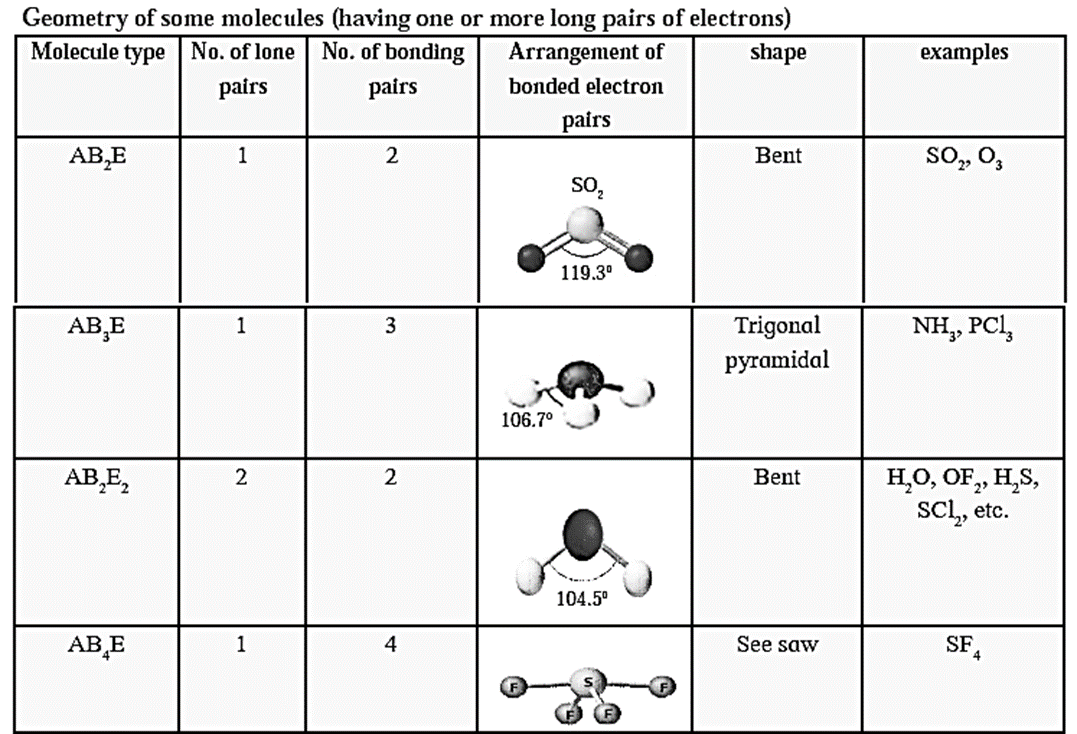
Valence Shell Electron Pair Repulsion Theory (VSEPR) :
- Sidgwick and Powell proposed valence shell electron pair repulsion theory to predict the shapes of covalent molecules.
- It is based on idea that the electron pairs on the atoms in a molecule repel each other.
- The atoms in a molecule arrange themselves in such a way that repulsion is minimised.
- The arrangement of electrons is called electron pair geometry.
Rules of VSEPR :
- Electron pairs arrange themselves in such a way that repulsion between them is minimum.
- The molecule acquires minimum energy and maximum stability.
- Lone pair of electrons also contribute in determining the shape of the molecule.
- Repulsion of other electron pairs by the lone pair (L.P) stronger than that of bonding pair (B.P)
Trend for repulsion between electron pair is
L.P − L.P > L.P − B.P > B.P − B.P
Lone pair − Lone pair repulsion is maximum because this electron pair is under the influence of only one nucleus while the bonded pair is shared between two nuclei.
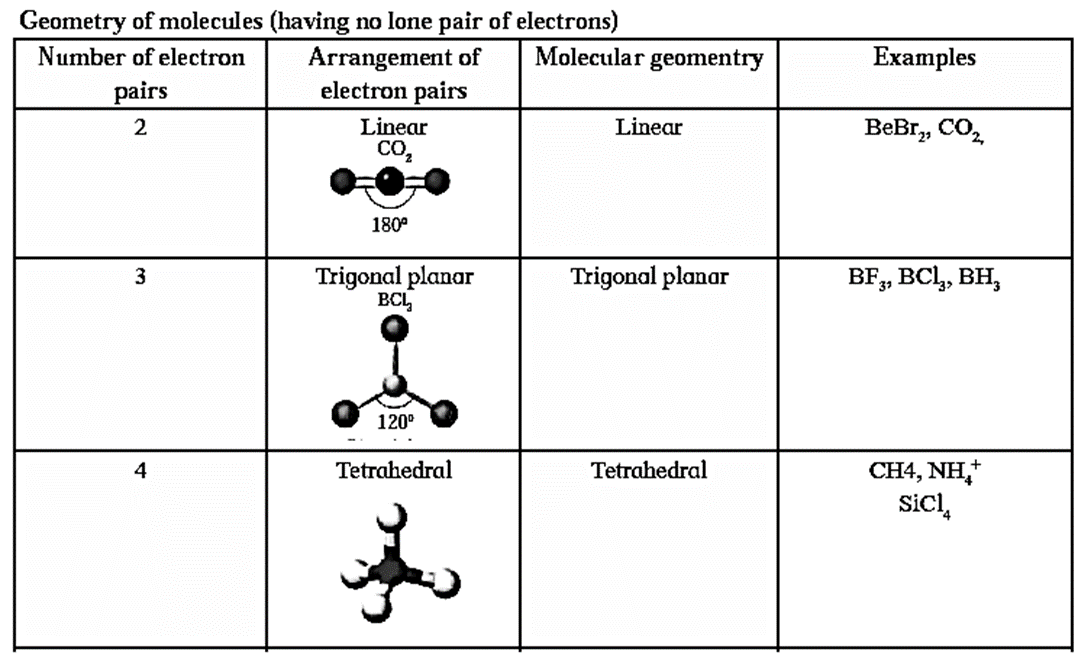
Thus the number of lone pair and bonded pair of electrons decide the shape of the molecules.
Molecules having no lone pair of electrons have a regular geometry. Depending on the number of lone pair and bonded pairs of electrons the molecules can be represented as AB2E, AB3E, AB2E2, AB4D, AB3E2, AB5E, AB4E2 where A is the central atom, B -bonded atom E - lone pair of electrons.
Main Page : – Maharashtra Board Class 11th-Chemistry – All chapters notes, solutions, videos, test, pdf.
Previous Chapter : Chapter-4-Structure of Atom – Online Notes
Next Chapter : Chapter-6-Redox Reactions – Online Notes

We reply to valid query.