Hydrocarbons
Maharashtra State Board-Class-11-Science-Chemistry-Chapter -15
Notes Part-1
|
Topics to be Learn : Part-1
|
Introduction :
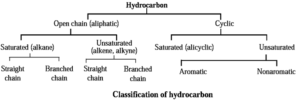
The compounds which contains carbon and hydrogen as the only elements are called hydrocarbons.
- Hydrocarbons can be open chain or cyclic.
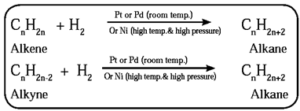
- In accordance with presence or absence of carbon-carbon multiple bond (C = C and / or C ≡ C) the hydrocarbons are called unsaturated or saturated.
- Cyclic unsaturated hydrocarbon can be either aromatic or nonaromatic.
Alkanes :
Alkanes are aliphatic saturated hydrocarbons containing carbon-carbon and carbon-hydrogen single covalent bonds.
- They are chemically less reactive and do not have much affinity for other chemicals. Hence, they are called paraffins.
- Alkanes have general formula CnH2n+2
Isomerism in alkanes :
The isomerism in alkanes is due to the difference in the structure of chains of carbon atoms. Hence, it is called structural isomerism and the isomers are called chain isomers.
Examples :
(i) Butane ((C4H10) has following chain isomers.
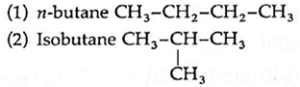
The two isomers have different physical and chemical properties.
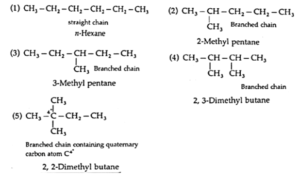
(ii) Structures of all the chain isomers of the saturated hydrocarbon containing six carbon atoms. With IUPC name.
Conformations in alkanes :
Conformation : Conformation refers to the phenomena of distinct spatial arrangements (three-dimensional arrangements in space) of atoms that can be freely rotated about C—C single bonds to transform into one—another. The compounds that exhibit this phenomenon are known as conformers or rotamers.
- These conformational isomers interconvert rapidly and cannot be isolated easily.
Example :
Conformations of ethane :
Ethane (C2H6) has two carbon atoms joined by single covalent bond. Each carbon atom has three hydrogen atoms.
Considering free rotation of one of the carbon atom around the C—C bond axis, infinite number of spatial arrangements of hydrogen atoms (attached to one carbon atom with respect to the hydrogen atoms attached to another carbon atom) are possible. These are called conformational isomers.
As they all have nearly the same energy, they can change from one form to another freely.
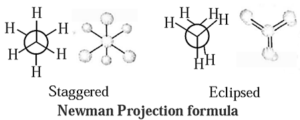
Thus two extreme arrangements are considered : viz. staggered and eclipsed conformations.
- Staggered conformation : In this, arrangement hydrogen atoms attached to two carbon atoms are as far apart as possible.
- Eclipsed conformation : In this, arrangement hydrogen atoms attached to two carbon atoms are as close as possible.
- The intermediate conformations during rotation are called skew conformation.
Representation of conformation : This conformation is represented by the following two projections (a) Sawhorse projection and (b) Newman projection.
(a) Sawhorse projection of ethane : In this representation, C—C bond is viewed from an oblique angle which indicates spatial arrangements by showing all C—H bonds.
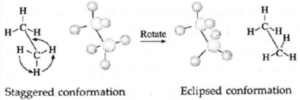
Newman projection of ethane : In this representation, C—C bond is viewed directly end—on and represents two carbon atoms by a circle. In this, lines drawn from the circle's center indicate bonds attached to the front carbon atom, and lines drawn from the circle's edge indicate bonds tied to the rear carbon atom.
Industrial preparation of alkanes :
(i) Industrial source : Crude petroleum and natural gas are the source of alkanes. Alkanes are obtained by fractional distillation of crude oil in oil refineries.
(ii) Methods of preparation of alkanes :
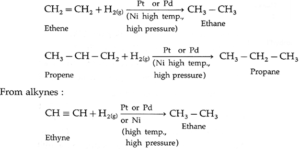
(1) From unsaturated hydrocarbons : Unsaturated hydrocarbons like alkenes,
alkynes on catalytic reduction by hydrogen gas in the presence of finely divided powder of platinum (Pt) or palladium (Pd) gives corresponding alkane
From alkenes :
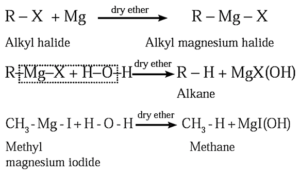
(2) From alkyl halides
(a) By reduction of alkyl halides : Alkyl halides on reduction with zinc and dilute hydrochloric acid form alkanes.
- The reduction of alkyl halides is due to nascent hydrogen obtained from the reducing agent Zn and dilute HCl.
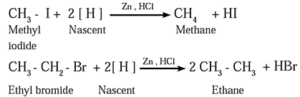
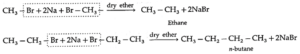
(b) By using reactive metal : When an alkyl halide is heated with sodium metal in dry ether, the corresponding higher alkane is formed. This is called Wartz reaction or Wartz coupling reaction.
(c) By using Grignard reagent (Alkyl magnesium halide) : When an alkyl halide is treated with magnesium metal in the presence of dry ether, alkyl magnesium halide is formed, which on further hydrolysis forms an alkane.
| Know This :
Preparation of Grignard reagent is an exothermic reaction. Hence no heating is required. In this reaction :
|
Physical properties of alkanes :
- Alkanes are colourless and odourless.
- At room temperature, first four alkanes (C1 to C4) are gases, C5 to C17 are liquids and higher alkanes C18 onwards are solids.
- Alkanes are insoluble in water but dissolve in non-polar solvents like benzene, ether, chloroform, etc.
- The branched chain alkanes have lower boiling points than straight chain alkanes. As the branches in alkanes increase, the boiling point lowers, because then the surface area decreases resulting in weaker intermolecular forces.
- Alkanes are nonpolar hence hydrophobic in nature.
Note : It is observed that polar substances are soluble in polar solvents where as nonpolar substances are soluble in nonpolar solvents. Alkanes being nonpolar in nature, they are insoluble in water and readily soluble in nonpolar solvent (organic solvent) like chloroform or ether.
Chemical properties of alkanes :
Alkanes are relatively unreactive towards acids, bases, oxidizing and reducing agents. They undergo the following reactions under specified conditions.
(1) Halogenation of alkanes :
Alkanes react with halogens in the presence of UV light or diffused sunlight or at higher temperature (573 to 773 K) to give mixture of alkyl halides.
Examples:
(i) Chlorination of methane :
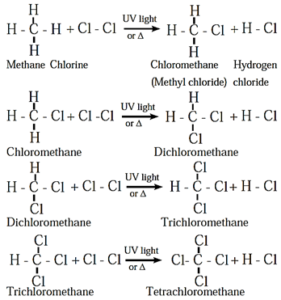
- Tetrachloromethane is a major product when excess of chlorine is used.
- Chloromethane is obtained as major product when excess of methane is employed.
- The reactivity of halogens toward alkanes follows the order F2 > Cl2 > Br2 > I2.
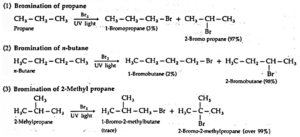
(ii) Bromination : Bromination gives corresponding bromides, but in different proportions.
Free radical mechanism : Halogenation of alkanes follows the free radical mechanism. Homolysis of halogen molecule (X2) generates halogen atoms, that is, halogen free radicals.
Example :
Mechanism of chlorination of methane : Methane reacts with chlorine to form methyl chloride in the presence of ultraviolet light. Chlorination of methane is a chain reaction which takes place by free radical mechanism in the following stages.
(i) Chain initiation : Chlorine molecule absorbs energy from photons and Cl-Cl bond breaks homolytically to give free radicals of chlorine.
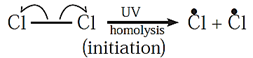
(ii) Chain propagation :
Step-i : Chlorine free radical being very reactive abstracts a hydrogen atom from methane to form methyl free radical and hydrogen chloride.
Step-ii : Methyl free radical thus formed attacks a chlorine molecule to give methyl chloride and a chlorine free radical.
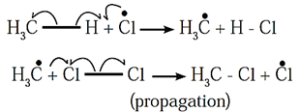 The chlorine free radical attacks methane (step i). The steps-i and ii are repeated many times and a chain of reactions is set up. The overall reaction is,
The chlorine free radical attacks methane (step i). The steps-i and ii are repeated many times and a chain of reactions is set up. The overall reaction is,
![]()
Remember :
- Depending upon which hydrogen atom is replaced, a number of isomeric products can be formed from alkane. For example, n-Butane and isobutane yield two isomers each.
- Halogenation an alkane yields a mixture of all possible isomeric products, indicating all the hydrogen atoms are susceptible to substitution.
(2) Combustion of alkanes :
Alkanes on heating in the presence of air or dioxygen are completely oxidized to carbon dioxide and water with the evolution of a large amount of heat.
This is an exothermic reaction. The amount of heat liberated due to combustion of one gram of a substance is called calorific value. Alkanes are good fuels
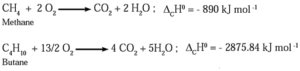
In general, combustion of alkane is represented
![]()
(3) Pyrolysis of alkanes :
The decomposition of alkanes at higher temperature, in absence of air to form lower alkanes, alkenes and hydrogen is called pyrolysis of alkanes or cracking.
The nature of products depend upon the structure of alkanes, presence or absence of catalyst and pressure.
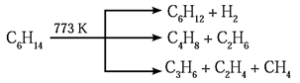
(4) Reforming :
Straight chain alkanes containing 6 to 10 carbon atoms are converted to benzene and its homologues on heating under 10 to 20 atm pressure at about 773 K in the presence of V2O5, Cr2O3, Mo2O3 etc. supported over alumina. The reaction involves simultaneous dehydrogenation and cyclization. This reaction is known as aromatization or reforming.
Examples :
(a)

(b) n-Heptane gets cyclised forming methyl benzene :

- This process is used in refineries to produce high quality gasoline (the fuel used in automobiles).
Uses of alkanes :
- First four alkanes are used as a fuel mainly for heating and cooking purpose. for example, LPG and CNG.
- CNG, petrol, diesel are used as fuels for automobiles.
- Lower liquid alkanes are used as solvent.
- Alkanes with more than 35 C atoms (tar) are used for road surfacing.
- Waxes are high molecular weight alkanes. They are used as lubricants. They are also used for the preparation of candles and carbon black that is used in manufacture of printing ink, shoe polish, etc.
Alkenes :
Alkenes : Aliphatic unsaturated hydrocarbons containing one or more double bonds are called alkenes (or olefins).
Example : CH2 = CH2.
The general formula of alkenes is CnH2n, where n is number of carbon atom.
Know This :
- Alkenes are also known as olefins, because the first member ethylene or ethene reacts with chlorine to form oily substance.
- The aliphatic unsaturated hydrocarbon containing two or three carbon-carbon double bonds are called alkadienes and alkatrienes, respectively. for example,
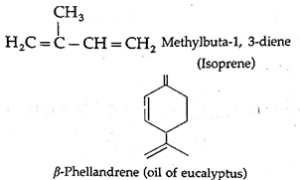
Isomerism in alkenes :
Alkenes with more than three carbon atoms show both structural isomerism and geometrical isomerism.
(i) Structural isomerism :
Structural isomerism in alkenes is of two types as follows :
(a) Chain isomerism : The isomerism arising due to the difference in carbon chain length is called chain isomerism.
(b) Position isomerism: The isomerism arising due to the difference in position of double bond in the same carbon chain is called position isomerism
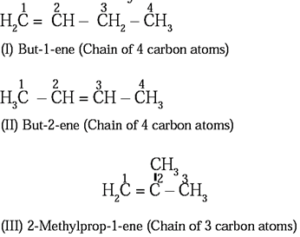
Structure I and III along with II and III are the examples of chain isomerism. They differ in carbon chain length. Structure I and II are the examples of position isomerism because they differ in the position of double bond in the same carbon chain.
(ii) Geometrical isomerism :
The isomerism which arises due to the difference in spatial arrangement of atoms or groups about doubly bonded carbon (C = C) atoms is called geometrical isomerism. There are two types of geometrical isomers as follows :
(a) Cis-isomer : The geometrical isomer in which two identical or similar atoms or groups lie on the same side of the double bond is called cis-isomer.
(b) Trans-isomer : The geometrical isomer in which two identical or similar atoms or groups lie on the opposite side of the double bond is called trans-isomer.
Example :
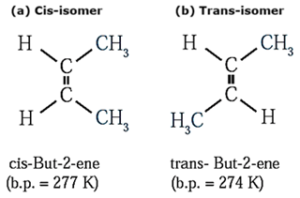
Properties of geometrical cis and trans-isomer :
- Cis and trans geometrical isomers have different physical properties like melting point, boiling point, etc.
- They have similar chemical properties.
- Their reactivities may be different.
- In cis-isomers due to spatial crowding of atoms or groups of atoms, there arises intramolecular repulsion. Hence, trans-isomer is more stable than cis—isomer.
| Know This :
· No terminal alkenes i.e. those with C=CH2 unit can exist as cis- and transisomers. · No 1,1-disubstituted alkenes i.e. those with C = CR2 unit can exist as cis- and trans -isomers. · Alkenes with general formulae RCH=CHR, R1R2C=CR1R2, R1CH=CR1R2, R1CH=CR2R3, R1CH=CHR2 and R1R2C=CR3R4 exhibit cis-trans isomerism. |
Preparation of alkenes :
(a) Industrial sources :
- Alkenes containing upto four carbon atoms can be obtained in pure form from the petroleum products.
- Ethene is produced from natural gas and crude oil by cracking.
(b) Methods of preparation of alkenes :
(1) Elimination reactions (1,2 -elimination ) : The reactions in which two atoms or groups are eliminated or removed from adjacent carbon atoms are called 1,2 - elimination reactions.
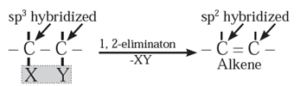
Some elimination reactions useful to prepare alkenes are :
(i) Dehydrohalogenation of alkyl halides : The reaction in which halogen atom from ∝-carbon atom and hydrogen atom from β-carbon atom (i.e., from the adjacent carbon atom) are eliminated is called dehydrohalogenation reaction. It is also called β -elimination reaction.
When an alkyl halide is boiled with a hot concentrated alcoholic solution of a strong base like KOH or NaOH, alkene is formed with removal of water molecule.
Examples :
(I) When ethyl bromide (bromo ethane) is boiled with alc.KOH, ethene is formed.
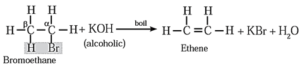
(II) When 2-Chlorobutane is boiled with alc. KOH, but-2-ene (major product) is formed.
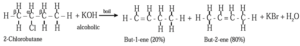
Saytzeff rule : In dehydrohalogenation the preferred product is the alkene that has the greater number of alkyl groups attached to doubly bonded carbon atoms.
The ease of dehydrohalogenation of alkyl halides accordingly is in the order 30 > 20 > 10
The ease of formation of alkenes :
R2C = CR2 > R2C= CHR > R2C = CH2, RCH=CHR > RCH=CH2
The stability of alkenes :
R2C=CR2 > R2C=CHR > R2C=CH2, RCH=CHR > RCH=CH2 > CH2=CH2
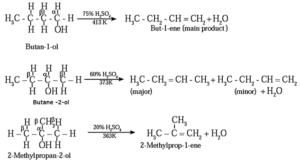
(ii) Dehydration of alcohol : The removal (elimination) of water molecule from a molecule of an alcohol is called dehydration of alcohol. Alcohole, in presence of a dehydrating agent undergoes β-elimination reaction to form an alkene.
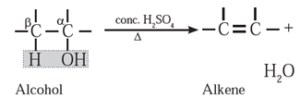
Example : Ethyl alcohol in presence of 95 % sulphuric acid at 443 K— 453 K or alumina at 623 K forms ethylene :
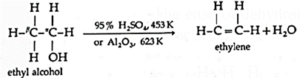
The ease of dehydration of alcohols is in the order 30 > 20 > 10
Examples :
Structures of alkenes obtained on dehydration of (1) Butan-1-ol (2) Butan-2-ol (3) 2-Methyl propan-2-ol
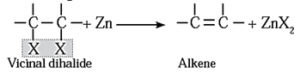
(iii) By dehalogenation of vicinal dihalides :
- Removal of two halogen atoms from adjacent carbon atoms is called dehalogenation.
- The dihalides of alkane in which two halogen atoms are attached to adjacent carbon atoms are called vicinal dihalides.
- Vicinal dihalides on heating with zinc metal form alkenes.
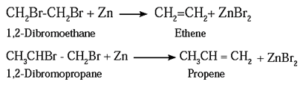
Examples :
Ethene and propene prepared from vicinal dihalide : (1) Ethylene dibromide (1,2-Dibromoethane) on heating with zinc in alcohol, forms ethane. (2) Propylene dichloride (1,2-Dichloropropane) on heating with zinc in alcohol forms propene
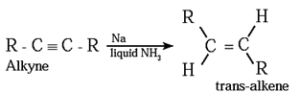
(2) Addition reactions : By partial reduction of alkynes (or controlled hydrogenation of alkynes) :
The C ≡ C triple bond of alkynes can be partially reduced to a C = C double bond with calculated quantity of dihydrogen in presence of Lindlar's catalyst (palladised charcoal deactivated partially with quinoline or sulfur compound).
The Cis isomer is obtained by this method.
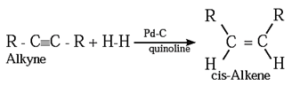
Trans alkene is obtained from alkyne on reduction with sodium in liquid ammonia.
Examples :
(i) Ethyne on partial reduction by H2 gas in the presence of Lindlar's catalyst (Pd/ C quinoline) forms ethene.
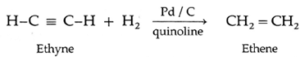
(ii) Propyne on partial reduction by H2 gas in the presence of Lindlar’s catalyst (Pd / CaCO3, quinoline) forms propene.
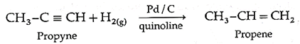
(iii) But-2-yne on partial reduction H2 gas in the presence of sodium in liquid ammonia form trans-but-2-ene.
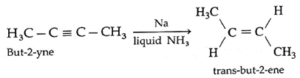
Physical properties of alkenes :
- Alkenes are nonpolar or weakly polar compounds those are insoluble in water, and soluble in nonpolar solvents like benzene, ether, chloroform.
- First three alkenes, Ethylene, propylene and or-butylene are gases. Next few members are liquids. Higher members are solids.
- Except ethylene, all other alkenes are colourless and odourless. Ethylene is a colourless gas with sickly sweet odour.
- Boiling point of alkenes increase with increase in molecular mass. A rise of 20 to 30 degree is observed for each added carbon atom.
- As in alkanes, alkenes with branching lowers boiling points.
Chemical properties of alkenes :
Alkenes are more reactive than alkene due to the presence of pi (π) electrons. They undergo electrophilic addition reactions.
The different reactions of alkenes are :
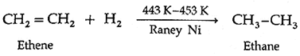
(i) Addition of dihydrogen/hydrogenation : Alkenes on catalytic hydrogenation at about 443 K—453 K under pressure in the presence of Raney Ni form alkanes.
Example :
(ii) Addition of halogens/halogenation : Alkenes are converted into the corresponding vicinal dihalides by addition of halogens (X2 = Cl2 or Br2) .
Example :
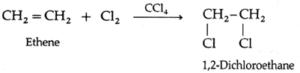
Alkenes on halogenation in the presence of CCl4 form vicinal dihalide. The reactivity of halogens is Cl2 > Br2 > I2.
(a) Ethene reacts with chlorine in the presence of CCl4 to form 1, 2-dichloroethane
(b) Propene reacts with bromine, the red brown colour of bromine disappears in CCl4 since 1, 2-dibromopropane is formed by halogenation reaction.

(iii) Addition of hydrogen halides/ hydrohalogenation : Addition of hydrogen halides (HX) like hydrogen chloride, hydrogen bromide and hydrogen iodide converts alkene into the corresponding alkyl halides.
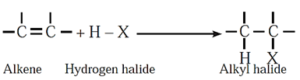
Example :
(i) Ethene on reaction with hydrogen bromide forms ethyl bromide
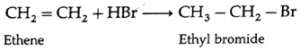
- The addition of HBr to symmetrical alkenes yield only one product.
Markovnikov’s rule: When an unsymmetrical reagent is added to an unsymmetrical alkene, the negative part of the reagent gets attached to that carbon atom of the double bond which carries less number of hydrogen atoms.
Example :
Addition of HBr to unsymmetrical alkene like propene gives two isomeric products.
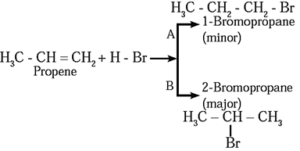
- Isopropyl bromide is the major product, since the negative part (Br—) of HBr is attached to carbon atom of a double bond with less number of hydrogen atoms.
Anti- Markovnikov addition Or peroxide effect or Kharasch - Mayo effect :
The addition of HBr to an unsymmetrical alkene (propene) in the presence of organic peroxide (R-O-O-R) takes place in the opposite orientation to that of Markovnikov’s rule and this is known as Kharasch-Mayo effect or peroxide effect or Anti-Markovnikov addition.
Example :
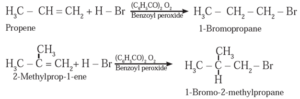
In the presence of peroxide, HBr to 2-Methyl prop-1—ene forms 1-Bromo-2-methylpropane.
| Remember :
· The orientation of addition of HBr to unsymmetrical alkene is determined by the presence or absence of peroxide. · The peroxide has no effect upon the addition of HCl and HI. |
(iv) Addition of sulfuric acid : Alkenes react with cold concentrated sulphuric acid to form alkyl hydrogen sulfate ( ROSO3H). The addition takes place according to Markovnikov's rule.
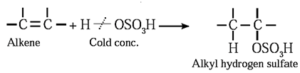
Examples :
(1) Ethene reacts with cold, concentrated sulphuric acid forming ethyl hydrogen sulphate, which on boiling with water forms ethyl alcohol.
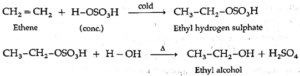
(2) Propene reacts with cold, concentrated sulphuric acid forming isopropyl hydrogen sulphate, which on boiling with water forms isopropyl alcohol. The addition of water takes place according to Markovnikov's rule. This reaction is known as hydration of alkenes.
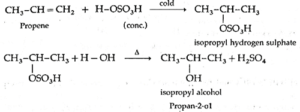
Q. Why Propan-1-ol and 2-Methylpropan-1-ol is not prepared by this method?
Answer : Reactive alkenes like propene or 2—methyl prop-1-ene on adding water molecules in the presence of conc. H2SO4 form alcohol. The addition of water takes place according to Mark0vnikov’s rule. Therefore, propan-1-ol and 2-methylpropan-1-ol cannot be prepared by this method.
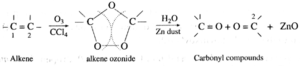
(v) Ozonolysis of alkenes : When a steam of ozonised oxygen is passed through a solution of an alkene in an inert solvent like CCl4, an unstable cyclic compound, ozonide is formed. Ozonide is treated with water in the presence of a reducing agent Zinc dust to form carbonyl compounds. This process is known as ozonolysis.
Examples :
Ozonolysis reaction for (1) ethene (2) propene :
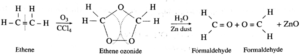
(1) When ozone gas is passed through a solution of ethene in an inert solvent CCl4, an unstable ethane ozonide is formed which on reduction with Zinc dust and water forms formaldehyde.
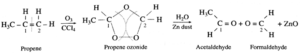
(2) When ozone gas is passed through a solution of propene in an inert solvent CCl4 an unstable ethane ozonide is formed, which on reduction with zinc dust and water forms acetaldehyde and formaldehyde
| Remember :
· In the cleavage products a carbonyl group ( C =O) is formed at each of the original doubly bonded carbon atoms. · Knowing the number and arrangement of carbon atoms in these aldehydes and · ketones produced, we can identify the structure of original alkene. · The role of zinc dust is to prevent the formation of hydrogen peroxide which oxidizes aldehydes to corresponding acids. · This reaction is used to locate the position and determine the number of double bonds in alkenes. |
(vi) Hydroboration- oxidation of alkene : When diborane is treated with alkene in the presence of tetrahydrofuran (THF) solvent, an addition product trialkyl borane is formed. Trialkyl borane is then oxidised with alkaline peroxide forms primary alcohol.
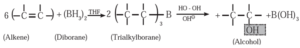
The addition of diborane to the double bond takes place in such a way that the boron gets attached to the less substituted carbon. The overall reaction gives Anti-Markovnikov’s product from unsymmetrical alkenes.
Examples :
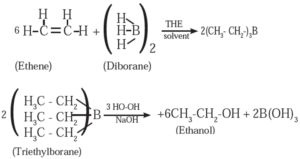
(1) When diborane is treated with ethene in the presence of THF, an addition product triethylborane is formed. Triethylborane is then oxidised with alkaline peroxide to form ethyl alcohol (ethanol).
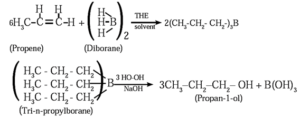
(2) When diborane is treated with propene in the presence of THF, an addition product tripropyl borane is formed. Tripropyl borane is then oxidised with alkaline peroxide to form propan-1-ol.
(vii) Polymerization : The process in which large number of small molecules join together and form very large molecules with repeating units is called polymerization.
- The compound having very large molecules made of large number repeating small units is called polymer
- The simple compound forming the repeating units in the polymer is called monomer.
Example : Ethene at high temperature and under high presssure interacts with oxygen, and undergoes polymerization giving high molecular weight polymer called polyethene.

Here n represents the number of repeating units and is a large number.
(viii) Hydroxylation : Alkenes react with cold and dilute alkaline potassium permanganate (KMnO4) to form glycols.
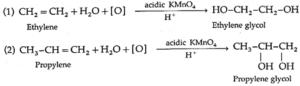
During this reaction the purple colour of KMnO4 disappears. Hence such reaction serves as qualitative test for detecting the presence of double bond in the compound under test. This is known as Baeyer's test.
Uses of alkenes :
- Ethene, in combination with oxygen is used in oxyethylene flame which is used for cutting and welding.
- Ethene may be used as anesthetic.
- Alkenes, are used as starting materials for preparation of alkyl halides, alcohols, aldehydes, ketones , acids etc.
- Ethene and propene are used to manufacture polythene, polypropylene those find use in bags, toys, bottles, etc.
- Ethene is used for artificial ripening of fruits, such as mangoes.
PDF : Class-11-Chemistry-Chapter-15-Hydrocarbons-Text Book
PDF : Class-11-Chemistry-Chapter-15-Hydrocarbons- Notes
PDF : Class-11-Chemistry-Chapter-15-Hydrocarbons-Solution
All 16 Chapters Notes -11-Chemistry-(16 PDF) Rs.132
All 16 Chapters-Solutions-11-Chemistry- (16 PDF) Rs.128
All 16 Chapters-Notes+Solutions-11-Chemistry- (32 PDF) Rs.228
Main Page : – Maharashtra Board Class 11th-Chemistry – All chapters notes, solutions, videos, test, pdf.
Previous Chapter : Chapter-14-Basic Principles of Organic Chemistry – Online Notes
Next Chapter : Chapter-16-Chemistry in Everyday Life – Online Notes