Is Matter Around Us Pure?
NCERT-Class-9-Science-Chapter-2
Notes
|
Topics to be learn :
|
Grocery items like oil, ghee, and milk are labeled 'pure' in grocery shops, but they are not pure from a scientific standpoint as they are mixtures of different substances.
What is a Mixture? :
A mixture is a substance containing two or more pure substances without any chemical combination, such as sea water, minerals, soil, or air.
- Dissolved sodium chloride can be separated from its solution in water through evaporation, but it is a pure substance.
- Sugar, on the other hand, contains only one kind of pure matter and its composition remains constant throughout.
Types of mixtures :
Depending upon the nature of the components that form a mixture, we have following two types of mixtures.
(i) Homogeneous Mixture :
A mixture in which the constituents are uniformly distributed throughout, i.e. without any clear boundary of separation, is called homogeneous mixture.
- The composition of a homogeneous mixture is uniform throughout.
- Some of the examples of homogeneous mixtures are salt solution, sugar solution, air, soft drinks, petroleum, biogas, alloys etc.
- Note Air is a homogeneous mixture of gas. Its two major constituents are oxygen (21%) and nitrogen (78%) and other gases in small quantities.
(ii) Heterogeneous Mixture :
A mixture that does not have uniform composition, i.e. has visible boundaries of separation between its constituents is called heterogeneous mixture.
- Here, the constituents of a heterogeneous mixture can be seen by naked eyes or under a microscope.
- Some examples of heterogeneous mixtures are sugar and sand mixture, salt and sand mixture, polluted air, muddy water etc.
What is a Solution?
Solution : A homogeneous mixture of two or more substances is called solution. A solution is sometimes also called a true solution.
- Lemonade, soda water, salt solution, sugar solution etc., all are the examples of solutions.
- In a solution, there is homogeneity at the particle level, i.e. the particles of dissolved substances are evenly distributed in the solution and are indistinguishable from one another.
There are two main components of a solution.
- Solvent (Dissolving phase) : The component (usually present in larger amount) of the solution that dissolves the other component in it, is called the solvent.
- Solute (Dissolved phase) : The component (usually present in lesser quantity) of the solution that is dissolved in the solvent is called the solute.
Some common examples of solution :
- In sugar solution, sugar is the solute and water is the solvent.
- A solution of iodine in alcohol known as tincture of iodine, has iodine (solid) as the solute and alcohol (liquid) as the solvent.
- Aerated drinks like soda water etc., are gas in liquid solutions. CO2(gas) as solute and water (liquid) as solvent.
- Solid solutions (alloys) and gaseous solutions (air).
| Know This :
Alloys : Alloys are mixtures of metals or non-metals that cannot be separated physically. They display their properties and can have variable compositions, such as brass, which is a mixture of 30% zinc and 70% copper. Despite their inability to be separated, alloys are considered mixtures. |
Properties of a Solution
- Some important properties of a solution are as follows:
- A solution is a homogeneous mixture.
- The particles of a solution are smaller than 1 nm (10-9 m) in diameter. Therefore, they cannot be seen by naked eyes.
- Due to very small particles, they do not scatter a beam of light passing through the solution. So, the path of light is not visible in a solution.
- A solution is stable, i.e. the solute particles do not settle down when left undisturbed. The solute particles cannot be separated from the mixture by the process of filtration.
Concentration of a solution :
The concentration of a solution is the amount of solute mass or volume present in a given amount (mass or volume) of solution.
In a solution, the relative proportion of the solute and solvent can be varied.
Solution with higher solute concentration is called concentrated solution and those with low concentration is called dilute solution.
Depending upon the amount of solute present in a given amount of solvent, the solution can be classified as
(i) Saturated solution : A saturated solution is a solution that dissolves as much solute as it can, meaning it cannot dissolve more solute at a specific temperature.
- The amount of the solute present in the saturated solution at this temperature is called its solubility.
(ii) Unsaturated solution : If the amount of solute contained in a solution is less than the saturation level, it is called an unsaturated solution.
Effect of Temperature on Solubility :
- Generally solubility of solids in liquids increases with increase in temperature and vice-versa.
- Generally solubility of gases in liquids increases with decrease in temperature and vice-versa.
Expressing the concentration of a solution : There are various ways of expressing the concentration of a solution,
(i) Mass by mass percentage of a solution = \(\frac{\text{Mass of Solute}}{\text{Mass of Solution}}\) × 100
(ii) Mass by volume percentage of a solution = \(\frac{\text{Mass of Solute}}{\text{Volume of Solution}}\) × 100
(iii) Volume by volume percentage of a solution = \(\frac{\text{Volume of Solute}}{\text{Volume of Solution}}\) × 100
What is a suspension? :
A suspension is a heterogeneous mixture in which the solute particles do not dissolve, but remain suspended throughout the bulk of the medium,
- The insoluble particles in a suspension are called 'suspended particles', whereas the solvent is referred to as 'medium'.
Examples :
- A mixture of chalk powder in water,
- A mixture of sand in water,
- Amoke coming out of a chimney of a factory.
Properties of Suspension :
- Suspension is a heterogeneous mixture.
- Its particles can be seen by naked eyes.
- Its particles scatter a beam of light passing through it and make its path visible (Tyndall effect).
- It is unstable, i.e. the solute particles settle down when suspension is left undisturbed. They can be separated by the process of filtration. When the particles settle down, the suspension breaks and it does not scatter light any more.
What is a colloidal solution? :
A colloid (or colloidal solution) is a mixture that is actually heterogeneous but appears to be homogeneous as the particles are uniformly spread throughout the solution, e.g. milk, shaving cream, cheese, etc.
- Colloidal solutions are also called colloidal sols.
- The components of a colloidal solution are the dispersed phase and the dispersion medium.
- The solute-like component or the dispersed particles in a colloid form is the dispersed phase and the component in which the dispersed phase is suspended is known as the dispersion medium.
Properties of a Colloid :
- A colloid is a heterogeneous mixture.
- The size of particles of a colloid is too small to be individually seen by naked eyes.
- They are big enough to scatter a beam of light passing through it and make its path visible.
- They are quite stable. Particles do not settle down when a colloid is left undisturbed.
- Particles of colloid can pass through filter paper, therefore a colloid cannot be separated by filtration. However, they get separated by a special technique called centrifugation.
Tyndall Effect :
The scattering of light by colloidal particles is known as Tyndall effect after the name of the scientist who discovered this effect.
In a true solution, the solute particles are so small that they cannot scatter light falling on them. In a colloidal solution, the particles are big enough to scatter light.
Tyndall effect can also be observed in the following situations:
- When a fine beam of light enters a room through a small hole (due to scattering of beam of light by the particles of dust and smoke in air).
- When sunlight passes through the canopy of a dense forest (as the mist containing tiny droplets of water scatter it)

Types of Colloids
Colloids are classified according to the state (solid, liquid or gas) of the dispersion medium and the dispersed phase.
Common examples of colloids :
| Dispersed phase | Dispersing Medium | Type | Example |
| Liquid | Gas | Aerosol | Fog, clouds, mist |
| Solid | Gas | Aerosol | Smoke, automobile exhaust |
| Gas | Liquid | Foam | Shaving cream |
| Liquid | Liquid | Emulsion | Milk, face cream |
| Solid | Liquid | Sol | Milk of magnesia, mud |
| Gas | Solid | Foam | Foam, rubber, sponge, pumice |
| Liquid | Solid | Gel | Jelly, cheese, butter |
| Solid | Solid | Solid Sol | Coloured gemstone, milky glass |
Physical and Chemical Changes
Compound is formed as a result of some chemical change while the mixing of the constituents in a mixture involves a physical change. Here, these two types of changes are discussed below.
Physical Changes :
- The properties that can be observed and specified like colour, hardness, rigidity, fluidity, density, melting point, boiling point etc., are physical properties.
- The changes which occur without a change in composition and in chemical nature of the substance are called physical changes.
- The interconversion of states is a physical change, e.g. change of water in ice is a physical change because chemically, ice and liquid water both are same. Although ice, water and water vapour all look different and display different physical properties but chemically they are same.
Chemical Changes :
- In chemical changes, one substance reacts with another substance to undergo a change in chemical composition.
- Chemical changes bring a change in the chemical properties of matter and a new substance is obtained.
- A chemical change is also called a chemical reaction, e.g. both water and cooking oil are liquid, but their chemical characteristics are different. They differ in odour and inflammability.
- Oil burns in air whereas, water extinguishes fire, i.e. it is the chemical property of oil that makes it different from water.
- Burning is a chemical change. During burning of a candle, both physical and chemical changes take place.
Comparison between physical and chemical change :
| Physical change | Chemical change |
| No new chemical substance is formed in this change. | New substance is formed which have different chemical composition. |
| It is a temporary change. | It is a permanent change. |
| This change is easily reversible | This change is usually irreversible. |
| The mass of a substance does not alter in this change. | The mass of a substance does alter in this change. |
| Generally, physical change is accompanied by very small or no energy changes. | Generally, chemical change is a accompanied by energy changes. |
What are the Types of Pure Substances?
Pure substances : The substances from which the mixture are formed are pure substances which are made up of single form of matter.
Classification of matter :
On the basis of its physical state matter can be classified as solid, liquid and gas.
On the basis of chemical composition matter can be classify as
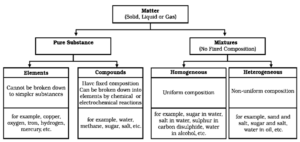
Pure Substances : A substance that consists of only a single type of constituent particles is called pure substance. It has fixed composition.
- e.g. gold, water etc. It cannot be separated into other kinds of matter by simple physical means.
Type of Pure Substances :
Based upon the nature of the constituent particles, it is of two types, i.e. elements and compounds.
Elements :
‘An element is a basic form of matter that contains only one kind of atoms and cannot be broken down into simpler substances by chemical reactions' e.g. Copper, oxygen, iron, hydrogen etc.
- There are total 118 elements have been discovered, out of these 92 are natural elements and others are man-made.
- Majority of elements are solid. 11 elements are in gaseous state at room temperature.
- Two elements i.e mercury and bromine are liquid at room temperature. However, gallium and cesium become liquid slightly above room temperature (303 K).
Classification of Elements :
On the basis of variation in properties, elements can be broadly classified as metals, non-metals and metalloids.
(i) Metals :
Properties of Metals :
- They are malleable, i.e. can be hammered into thin sheets.
- They are ductile i.e. can be drawn into thin wires.
- They are lustrous (shine).
- They have silver-grey or yellow colour.
- They are sonorous, i.e. make a ringing sound when hit.
- They conduct heat and electricity. e.g. Gold, silver, copper, mercury etc.
- Mercury is the only metal that is liquid at room temperature.
(ii) Non-metals :
Properties of Non-metals :
- They are poor conductors of heat and electricity.
- They display a variety of colours.
- They are not lustrous, sonorous or malleable. e.g. Hydrogen, oxygen, iodine, carbon (coal, coke), bromine, chlorine, etc.
- Bromine is the only non-metallic element that exists in liquid state at normal conditions of temperature and pressure.
(iii) Metalloids : Elements having intermediate properties between those of metals and non-metals are called metalloids. e.g. Boron, silicon, germanium, etc.
Compounds :
A compound is a substance composed of two or more elements, chemically combined with one another in a fixed proportion.
e.g. Water (H2O), methane (CH4), carbon dioxide (CO2), ammonia (NH3), sodium chloride (NaCl)
Mixtures and Compounds :
| Mixtures | Compounds |
| Elements or compounds just mix together to form a mixture and no new compound is formed. | Elements react to form new compounds. |
| A mixture has a variable composition. | The composition of each new substance is always fixed. |
| A mixture shows the properties of the constituent substances. | The new substance has totally different properties. |
| The constituents can be separated
fairly easily by physical methods. |
The constituents can be separated only by chemical or electrochemical
reactions. |
Click on below links to get PDF from store
PDF : NCERT-Class 9-Science-Chapter-2-Is Matter Around Us Pure?-Notes
PDF : NCERT-Class 9-Science-Chapter-2-Is Matter Around Us Pure?-Solution
Main Page : NCERT-Class-9-Science – All chapters notes, solutions, videos, test, pdf.
Previous Chapter : Chapter-1-Matter in our surroundings – Online Notes
Next Chapter : Chapter-3- Atoms and molecules – Online Notes
We reply to valid query.