Ionic Equilibria
Maharashtra Board-Class-12-Chemistry-Chapter-3
Solutions
Question 1. Choose the most correct answer :
(i) The pH of 10-8 M of HCl is
(a) 8
(b) 7
(c) less than 7
(d) greater than 7
(c) less than 7
(ii) Which of the following solution will have pH value equal to 1.0 ?
(a) 50 mL of 0.1M HCl + 50mL of 0.1M NaOH
(b) 60 mL of 0.1M HCl + 40mL of 0.1M NaOH
(c) 20 mL of 0.1M HCl + 80mL of 0.1M NaOH
(d) 75 mL of 0.2M HCl + 25mLof 0.2M NaOH
(d) 75 mL of 0.2M HCl + 25mLof 0.2M NaOH
(iii) Which of the following is a buffer solution ?
(a) CH3COONa + NaCl in water
(b) CH3COOH + HCl in water
(c) CH3COOH+CH3COONa in water
(d) HCl + NH4Cl in water
(c) CH3COOH+CH3COONa in water
(iv) The solubility product of a sparingly soluble salt AX is 5.2×10-13. Its solubility in mol dm-3 is
(a) 7.2 × 10-7
(b) 1.35 × 10-4
(c) 7.2 × 10-8
(d) 13.5 × 10-8
(a) 7.2 × 10-7
(v) Blood in human body is highly buffered at pH of
(a) 7.4
(b) 7.0
(c) 6.9
(d) 8.1
(a) 7.4
(vi) The conjugate base of [Zn(H2O)4]2+ is
(a) [Zn(H2O)4]2+NH3
(b) [Zn(H2O)3] 2+
(c) [Zn(H2O)3OH]+
(d) [Zn(H2O)H]3+
(c) [Zn(H2O)3OH]+
(vii) For pH > 7 the hydronium ion concentration would be
(a) 10-7M
(b) < 10-7M
(c) > 10-7M
(d) ≥ 10-7M
(b) < 10-7M
Question 2. Answer the following in one sentence :
(i) Why cations are Lewis acids ?
Because cations lack electrons, they receive a pair of electrons and are hence Lewis acids.
(ii) Why is KCl solution neutral to litmus?
- Since KCI is a salt of strong base KOH and strong acid HCl, it does not undergo hydrolysis in its aqueous solution.
- Due to strong acid and strong base, concentrations [H3O+] = [OH—] and the solution is neutral.
(iii) How are basic buffer solutions prepared?
- A basic buffer solution is made by combining aqueous solutions of a weak base, such as NH4OH, with its salt of a strong acid, such as NH4
- A weak base is chosen based on the needed pH or pOH of the solution and the weak base's dissociation constant.
(iv) Dissociation constant of acetic acid is 1.8 × 10—5. Calculate percent dissociation of acetic acid in 0.01 M solution.
Given : Ka = 1.8 x 10−5; C= 0.01 M, Percent dissociation = ?
Ka = \(\frac{Cα^2}{1-α}≈Cα^2\)
∴ α = \(\sqrt{\frac{K_a}{C}}\) = \(\sqrt{\frac{1.8×10^{-5}}{0.01}}\)
= 4.242 x 10-2
Per cent dissociation = α x 100
= 4.242 x 10-2 x 102
= 4.242 %
Answer is: Per cent dissociation = 4.242 %.
(v) Write one property of a buffer solution.
Properties of a buffer solution :
- The pH of a buffer solution is maintained appreciably constant.
- By addition of a small amount of an acid or a base pH does not change.
- On dilution with water, pH of the solution doesn’t change.
(vi) The pH of a solution is 6.06. Calculate its H+ ion concentration.
Given : pH = 6.06, [H+] = ?
pH = −log10 [H+]
log10 [H+] = −pH
[H+] = Antilog −pH
= Antilog −6.06
= Antilog .94
= 8.714 x 10−7 M
Answer is : [H+] = 8.714 x 10−7 M
(vii) Calculate the pH of 0.01 M sulphuric acid.
Given : C = 0.01 M H2SO4, pH = ?
H2SO4(aq) →2H+(aq) + SO42−(aq)
∴ [H3O+] = 2 x 0.01 = 0.02 M
pH = −log10[H3O+]
= − log10 0.02
= − ( .3010)
= 2 − 0.3010
= 1.6990
Answer is : pH = 1.6990.
(viii) The dissociation of H2S is suppressed in the presence of HCl. Name the phenomenon.
The weak dibasic acid H2S is dissociated as follows :
H2S (aq) + 2H2O (l) ⇌ 2H3O+ (aq) + S2— (aq)
The dissociation constant Ka is represented as,
Ka = \(\frac{[H_3O^+]^2[S^{2-}]}{[H_2S]}\)
When HCl is added, it increases the concentration of common ion H3O+.
HCl (aq) + H2O (l) → H3O+ (aq) + Cl— (aq)
Hence by Le Chaterlier’s principle, the equilibrium is shifted from right to left, suppressing the dissociation of weak electrolyte H2S.
(ix) Why is it necessary to add H2SO4 while preparing the solution of CuSO4?
- CuSO4 is a salt of strong acid H2SO4 and weak base Cu(OH)2.
- CuSO4 in aqueous solution undergoes hydrolysis and forms a precipitate of Cu(OH)2. And solution becomes turbid.
CuSO4 + 2H2O ⇌ Cu(OH)2↓ + H2SO4
OR
CuSO4 + 4H2O ⇌ Cu(OH)2 + 2H3O+ + SO42-
. turbidity
- When H2SO4 is added, the hydrolysis equilibrium is shifted to left hand side and Cu(OH)2 dissolves giving clear solution.
(x) Classify the following buffers into different types :
(a) CH3COOH + CH3COONa
(b) NH4OH + NH4Cl
(c) Sodium benzoate + benzoic acid
(d) Cu(OH)2 + CuCl2
(a) Acidic buffer (CH3COOH + CH3COONa)
(b) Basic buffer (NH4OH + NH4Cl)
(c) Acidic buffer (Sodium benzoate + benzoic acid)
(d) Basic buffer (Cu(OH)2 + CuCl2)
Question 3. Answer the following in brief :
(i) What are acids and bases according to Arrhenius theory ?
According to Arrhenius theory acids and bases are defined as follows :
Acid : Acid is a substance which contains hydrogen and gives rise to H+ ions in aqueous solution.
For example : HCl
HCl (aq) → H+(aq) + Cl−(aq)
CH3COOH(aq) \(\underleftrightarrow{water}\) CH3COO (aq)+ H+(aq)
Base : A substance that contains OH group and produces hydroxide ions (OH−) in aqueous solution is called a base.
For example : NaOH
NaOH(aq) → Na+(aq) + OH−(aq) .
NH4OH(aq) ⇌ NH4+(aq) + OH−(aq)
(ii) What is meant by conjugate acid-base pair?
Conjugate acid-base pair : A pair of an acid and a base differing by a proton is called a conjugate acid-base pair.
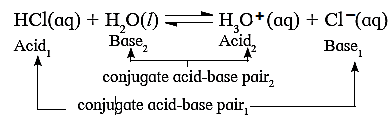
(iii) Label the conjugate acid-base pair in the following reactions
(a) HCl + H2O ⇌ H3O+ + Cl−
(b) CO32− + H2O ⇌ OH− + HCO3−
(a) HCl + H2O ⇌ H3O+ + Cl−
Acid-1 Base-2 Acid-2 Base-1
(b) CO32− + H2O ⇌ OH− + HCO3−
Base-1 Acid-2 Base-2 Acid-1
(iv) Write a reaction in which water acts as a base.
H2O(l) + HCl ⇌ H3O+ + Cl
Base—1 Acid—2 Acid-1 Base—2
Since water accepts a proton, it acts as a base
(v) Ammonia serves as a Lewis base whereas AlCl3 is Lewis acid. Explain.
- Since ammonia molecule, NH3 has a lone pair of electrons to donate it acts as a Lewis base.
- AlCl3 is a molecule with incomplete octet hence it is electron deficient and acts as a Lewis acid.
(vi) Acetic acid is 5% ionised in its decimolar solution. Calculate the dissociation constant of acid.
Given : C = 0.1 M; Dissociation = 5%, Ka = ?
α = \(\frac{Percent\,dissociation}{100}=\frac{5}{100}\) = 0.05
Ka = \(\frac{Cα^2}{1-α}\) = \(\frac{0.1×0.05^2}{1-0.05}\) = 2.63 x 10−4
Answers is : Dissociation constant of acid = Ka = 2.63 x 10−4
(vii) Derive the relation pH + pOH = 14.
Relationship between pH and pOH :
The ionic product of water, Kw is given by,
Kw = [H3O+] x [OH−]
At 298 K, Kw = 1 x 10−14.
pKw = −log10Kw = − log101 x 10−14.= 14
[H3O+] x [OH−] = 1 x 10−14
Taking logarithm to base 10 of both sides,
log10[H3O+] + log1o[OH−] = log101 x 10−14
Multiplying both the sides by − 1,
−log10[H3O+] − log1o[OH−] = −log101 x 10−14
‘.’ pH = —log10[H3O+], pOH = — log10[OH−]
pKw = −log10Kw
∴ pH + pOH = pKw
Or pH + pOH = 14
(viii) Aqueous solution of sodium carbonate is alkaline whereas aqueous solution of ammonium chloride is acidic. Explain.
(A) (i) Sodium carbonate is a salt of weak acid and strong base.
(ii) In aqueous solution it undergoes hydrolysis.
Na2CO3(aq) + 2H2O(l) ⇌ 2NaOH(aq) + H2CO3(aq)
salt strong base weak acid
2Na+(aq) + CO32− 2H2O(aq) ⇌ 2Na+(aq) + 2OH−(aq) + H2CO3(aq)
(iii) Strong base dissociates completely while weak acid dissociates partially hence [OH−] > [H3O+] and the solution is basic.
(B) (i) Ammonium chloride is a salt of strong acid and weak base.
(ii) In aqueous solution it undergoes hydrolysis.
NH4Cl(aq) + H2O(aq) ⇌ NH4OH(aq) + HCl(aq)
salt weak base strong acid
NH4+ +Cl−(aq) + H2O(aq) ⇌ NH4OH(aq) + H+ + Cl−(aq)
(iii) Since [H+] or [H3O+] > [OH−] the solution is acidic.
(ix) pH of a weak monobasic acid is 3.2 in its 0.02 M solution. Calculate its dissociation constant.
Given : pH = 3.2; C = 0.02 M; Ka = ?
K pH = −1og10 [H+]
[H+] = Antilog −pH
= Antilog −3.2
= Antilog .8
= 6.31 x 10−4 M
HA(aq) ⇌ H+ + A—
c(1 − α) cα cα
‘.’ [H+] = cα
α = \(\frac{H^+}{c}=\frac{6.31×10^{-4}}{0.02}\) = 0.0315
Ka = cα2 = 0.02 x (0.0315)2 = 1.984 x 10—5
Answer is: Dissociation constant = Ka = 1.984 x 10—5
(x) In NaOH solution [OH ] is 2.87 × 10—4. Calculate the pH of solution.
Given: [OH−] = 2.87 x 10−4 M, pH = ?
pOH = −log10[OH−]
= − log10 2.87 x 10−4
= −( .4579)
= (4 − 0.4579)
= 3.5421
pH + pOH = 14
pH =14 − pOH = 14 − 3.5421 = 10.4579
Answer is : pH = 10.4579.
Question 4. Answer the following :
(i) Define degree of dissociation. Derive Ostwald's dilution law for the CH3COOH.
Degree of dissociation : It is defined as a fraction of total number of moles of an electrolyte that dissociate into its ions at equilibrium. It is denoted by symbol ∝ and given by
α = \(\frac{\text{number of mole dissociated}}{\text{total number of moles}}\)
Percent dissociation = α × 100
If 'c' is the molar concentration of an electrolyte the equilibrium concentration of cation or anion is (α × c) mol dm—3.
Ostwald's dilution law for the CH3COOH.
Consider the dissociation of a weak electrolyte HA. Let V dm3 of a solution contain one mole of CH3COOH.
Then the concentration of a solution is, C = 1/V mol dm−3
Let α be the degree of dissociation of CH3COOH,
| HA ⇌ H+ + A | |||
| Initial moles : | 1 | 0 | 0 |
| Moles of equilibrium : | 1− α | α | α |
| Concentration at equilibrium (mol dm−3) | (1− α)/V | α /V | α /V |
Applying the law of mass action to this dissociation equilibrium, we have,
Ka = \(\frac{[CH_3COO^-]×[H^+]}{[CH_3COOH]}\)
∴ Ka = \(\frac{α/V\,×α/V}{(1-α)/V}=\frac{α^2}{(1-α)V}\)
‘.’ C = 1/V mol dm3
∴ Ka = \(\frac{Cα^2}{(1-α)}\)
This is the Ostwald’s dilution law.
(ii) Define pH and pOH. Derive relationship between pH and pOH.
- pH : The negative logarithm, to the base 10, of the molar concentration of hydrogen ions, H+ is known as the pH of a solution.
pH = − log10[H+]
- pOH : The negative logarithm, to the base 10, of the molar concentration of hydroxyl ions, OH− is known as the pOH of a solution.
pOH = −log10[OH−]
Relationship between pH and pOH :
The ionic product of water, Kw is given by,
Kw = [H3O+] x [OH−]
At 298 K, Kw =1 x 10−14.
pKw = −log10Kw= − log1o1 x 10−14.= 14
[H3O+] x [OH−] = 1 x 10−14
Taking logarithm to base 10 of both sides,
log10[H3O+] + log1o[OH−] = log1o1 x 10−14
−log10[H3O+] − log1o[OH−] = −log1o1 x 10−14 …(Multiplied both sides by −1)
‘.’ pH = −log10[H3O+], pOH = − log1o[OH−]
pKw = −log10Kw
∴ pH + pOH = pKw
Or pH + pOH = 14
(iii) What is meant by hydrolysis ? A solution of CH3COONH4 is neutral. why ?
Hydrolysis : A reaction in which the cations or anions or both the ions of a salt react with water to produce acidity or basicity or sometimes neutrality is called hydrolysis.
Consider hydrolysis of CH3COONH4.
CH3COO— + NH4+ H2O ⇌ CH3COOH + NH4OH
Salt weak acid weak base
Since Ka = Kb, the weak acid CH3COOH and weak base NH4OH dissociate to the same extent, hence, [H3O+] = [OH—] and the solution reacts neutral after hydrolysis.
(iv) Dissociation of HCN is suppressed by the addition of HCl. Explain.
The weak acid HCN is dissociated as follows :
HCN (aq) + H2O (l) ⇌ H3O (aq) + CN— (aq)
The dissociation constant Ka is represented as,
Ka = \(\frac{[H_3O^+]^2×[CN^-]}{[HCN]}\)
When HCl is added, it increases the concentration of H3O+, hence in order to keep the ratio constant, then by Le Chatelier’s principle, the equilibrium is shifted from right to left, suppressing the dissociation of HCN.
(vi) Derive the relationship between degree of dissociation and dissociation constant in weak electrolytes.
Consider the dissociation of a weak electrolyte HA. Let V dm3 of a solution contain one mole of the electrolyte.
Then the concentration of a solution is, C = 1/V mol dm−3
Let α be the degree of dissociation of the electrolyte,
| HA ⇌ H+ + A | |||
| Initial moles : | 1 | 0 | 0 |
| Moles of equilibrium : | 1− α | α | α |
| Concentration at equilibrium (mol dm−3) | (1− α)/V | α /V | α /V |
Applying the law of mass action to this dissociation equilibrium, we have,
K = \(\frac{[H^+]×[A^-]}{[HA]}\)
∴ K = \(\frac{α/V\,×α/V}{(1-α)/V}=\frac{α^2}{(1-α)V}\)
As the electrolyte is weak, ∝ is very small as compared to unity,
(1 — α) ≈ 1.
∴ K = \(\frac{α^2}{V}\)
∴ α = \(\sqrt{KV}\)
∴ α ∝ \(\sqrt{V}\)
1/V = C, where C = concentration in mol dm−3
∴ K = ∝2C
∴ α = \(\sqrt{\frac{K}{C}}\)
∴ α = \(\sqrt{KV}\) (‘ .’ C = 1/V or V = 1/C)
∴ α = \(\sqrt{\frac{K}{C}}\)
This is the expression of Ostwald’s dilution law. Thus, the degree of dissociation of a weak electrolyte is directly proportional to the square root of the volume of the solution containing 1 mole of an electrolyte.
(vii) Sulfides of cation of group II are precipitated in acidic solution (H2S + HCl) whereas sulfides of cations of group IIIB are precipitated in ammoniacal solution of H2S. Comment on the relative values of solubility product of sulfides of these.
- In qualitative analysis, the cations of group II are precipitated as sulphides, namely HgS, CuS, PbS, etc., while cations of group IIIB are precipitated as sulphides, namely, CoS, NiS, ZnS.
- The sulphides of group II have extremely low solubility product (Ksp) about 10−29 to 10−53 while the sulphides of group IIIB have slightly higher Ksp values about 10−20 to 10−30
- In group II, sulphides are precipitated by adding H2S in acidic solution while in IIIB group they are precipitated in a basic solution like ammonical solution.
- In acidic medium due to common ion H+, H2S is dissociated to very less extent but gives sufficient S2− ion to exceed solubility product of group II sulphides of cations and precipitate them.
HCl (aq) → H+ (aq) + Cl— (aq)
H2S (aq) ⇌ 2H+(aq) + S2— (aq)
- In basic medium, H+ from H2S are removed by OH— in solution, or by NH4OH, increasing the dissociation of H2S and concentration of S2—, so that IP > Ksp.
H2S (aq) ⇌ 2H+ (aq) + S2— (aq)
NH4OH(aq) + H+ (aq) → NH4+(aq) + H2O (l)
- Therefore, group II cations are precipitated in an acidic medium while cations of group IIIB are precipitated in ammonical solution.
(viii) Solubility of a sparingly soluble salt get affected in presence of a soluble salt having one common ion. Explain.
Consider the solubility equilibrium of a sparingly soluble salt, AgCl.
AgCl (s) ⇌ Ag (aq) + Cl— (aq)
The solubility product, Ksp is given by,
Ksp = [Ag+] x [Cl—]
Consider addition of a strong electrolyte AgNO3 with a common ion Ag+.
AgNO3(aq) _’ Aggq) + Noiaq)
The concentration of Ag+ in the solution is increased, hence by Le Chatelier’s principle the equilibrium of AgCl is shifted to left hand side since the value of Ksp is constant.
Thus in the presence of a common ion, the solubility of a sparingly soluble salt is suppressed.
(ix) The pH of rain water collected in a certain region of Maharashtra on particular day was 5.1. Calculate the H+ ion concentration of the rain water and its percent dissociation.
Given : pH = 5.1, [H+] = ?
pH = — log10 [H+]
log10 [H+] = −pH
[H+] = Antilog −pH
= Antilog −5.1
= Antilog .9
= 7.943 x 10−6 M
Answer is: [H+] = 8.714 x 10−7 M
(x) Explain the relation between ionic product and solubility product to predict whether a precipitate will form when two solutions are mixed?
- If ionic product and solubility product are indicated by IP and K(sp) respectively then,
- When IP = K(sp) the solution is saturated.
- When IP > K(sp) the solution is supersaturated and hence precipitation will occur, when two solutions are mixed.
- When IP < K(sp) the solution is unsaturated and precipitation will not occur, when two solutions are mixed.
PDF : Class-12-Chemistry-Chapter-3-Ionic Equilibria-Text Book
PDF :Class-12-Chemistry-Chapter-3-Ionic Equilibria- Notes
PDF :Class-12-Chemistry-Chapter-3-Ionic Equilibria- Solution
All 16 Chapters Notes -Class-12-Chemistry (16-PDF)
All 16 Chapters Solutions -Class-12-Chemistry (16-PDF)
All 16 Chapters Notes+Solutions -Class-12-Chemistry (32-PDF)
Main Page : – Maharashtra Board Class 12th-Chemistry – All chapters notes, solutions, videos, test, pdf.
Previous Chapter : Chapter-2-Solutions – Online Solutions
Next Chapter : Chapter-4-Chemical Thermodynamics – Online Solutions
We reply to valid query.