Halogen Derivatives
Maharashtra Board-Class-12-Chemistry-Chapter-10
Notes-Part-1
Topics to be Learn : Part-2
|
Chemical properties :
Laboratory test of haloalkanes :
Haloalkanes are of neutral type in aqueous medium. On warming with aqueous sodium or potassium hydroxide the covalently bonded halogen in haloalkane is converted to halide ion.
R-X + OH— \(\underrightarrow{Δ}\) R-OH + X—
When this reaction mixture is acidified by adding dilute nitric acid and silver nitrate
solution is added a precipitate of silver halide is formed which confirms presence of halogen in the original organic compound.
Ag+(aq) + X—(aq) → AgX↓ (s)
Nucleophilic substitution reactions of haloalkanes :
Substitution reaction : When a group bonded to a carbon in a substrate is replaced by another group to get a product with no change in state of hybridization of that carbon the reaction is called substitution reaction.
Nucleophilic substitution reactions (SN) : Alkyl halides react with a variety of nucleophiles to give nucleophilic substitution reactions. The reaction is represented in general form as shown below.
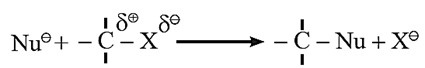
- When a substrate reacts fast it is said to be reactive.
- The reactivity of alkyl halides in SN reaction depends upon two factors, (i) the substitution state (10, 20 or 30) of the carbon and (ii) the nature of the halogen.
- The order of reactivity : tertiary alkyl halide (30) > secondary alkyl halide (20) >primary alkyl halide (10) and R - I > R - Br > R - Cl
Examples of some important nucleophilic substitution reactions of alkyl halides :
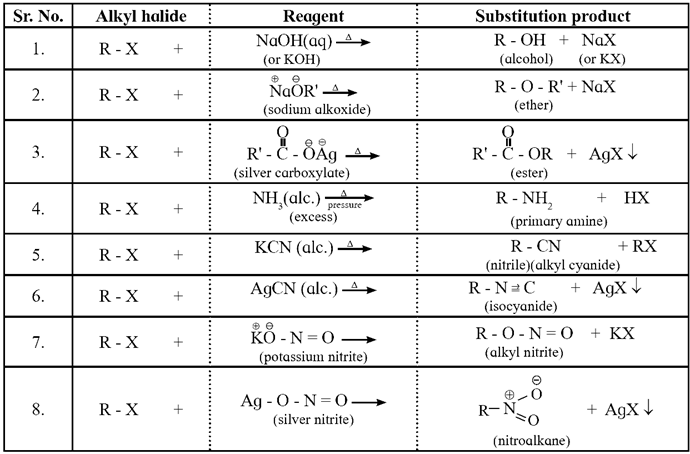
Examples :
Action of aqueous KOH (or Na0H) on :
(i) Ethyl bromide : When ethyl bromide (bromoethane) is refluxed with aqueous potassium hydroxide, ethyl alcohol is formed. The reaction is called a hydrolysis reaction.
CH3—CH2—Br + KOH \(\underrightarrow{boil}\) CH3—CH2—OH + KBr
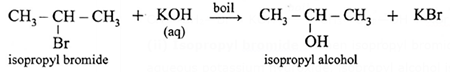
(ii) Isopropyl bromide : When isopropyl bromide (2-bromopropane) is boiled with aqueous potassium hydroxide, isopropyl alcohol is formed.
(iii) Methyl bromide : When methyl bromide (bromomethane) is heated with aq. KOH, it is hydrolysed to methyl alcohol (methanol).
CH3—Br + KOH \(\underrightarrow{boil}\) CH3—OH +KBr
(iv) Tert-butyl chloride : When tert-butyl chloride is refluxed with aqueous potassium hydroxide, tert-butyl alcohol is formed.
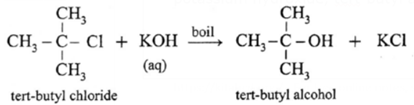
Williamson’s synthesis : When an alkyl halide (R—X) is heated with sodium alkoxide (R—O—Na), an ether is obtained. In this reaction halide (—X) of alkyl halide is replaced by an alkoxy group (—OR). This reaction is known as Williamson’s synthesis. This method is used to prepare simple (or symmetrical) ethers and mixed
(or unsymmetrical) ethers.
Sodium alkoxide is obtained by a reaction of sodium with an alcohol.
2R-OH + 2Na → 2R-O—Na+ + H2
e. g. 2C2H5OH + 2Na → 2C2H2O—Na+ + H2
Simple (symmetrical) ether : When an alkyl halide and sodium alkoxide having similar alkyl groups are heated, symmetrical ether is obtained.
R—O—Na + RX \(\underrightarrow{Δ}\) R-O—R + NaX
e.g. When ethyl bromide is heated with sodium ethoxide, diethyl ether is formed.
C2H5—O—Na + C2H5—Br \(\underrightarrow{Δ}\) C2H5—O—C2H5 + NaBr
Mixed (unsymmetrical) ether : When an alkyl halide and sodium alkoxide having different alkyl groups are heated, unsymmetrical ether is obtained.
R—O—Na + R'—X \(\underrightarrow{Δ}\) R—O—R' + NaX
When methyl bromide is heated sodium ethoxide, ethyl methyl ether is formed.
C2H5—O-Na + CH3—Br \(\underrightarrow{Δ}\) C2H5—O—CH3 + NaBr
When ethyl bromide is heated with sodium methoxide, ethyl methyl ether is formed.
CH3—O—Na + C2H5Br \(\underrightarrow{Δ}\) C2H5—O—CH3 + NaBr
Ammonolysis : When an alkyl halide is boiled under pressure with an excess of alcoholic solution of ammonia (NH3) corresponding (primary amine) alkyl amine is formed. This reaction is known as ammonolysis of alkyl halide.
R—X + NH3 \(\frac{413K}{pressure}\)> R—NH2 + H X
Action of excess of ammonia on,
(i) Ethyl bromide : When ethyl bromide is boiled under pressure with an excess of alcoholic ammonia, ethylamine (ethanamine) is formed. This is known as ‘ammonolysis’ of ethyl bromide.
CH3—CH2—Br + NH3 \(\frac{413K}{pressure}\)> CH3—CH2—NH2 + HBr
(ii) n-propyl bromide : When n-propyl bromide is boiled under pressure with an excess of ammonia, n-propyl amine (propanamine) is formed.
CH3—CH2—CH2—Br + NH3 \(\frac{413K}{pressure}\)> CH3—CH2—CH2—NH2 + HBr
| Know This :
Cyanide ion is capable of attacking through more than one site (atom).
Such nucleophiles are called ambident nucleophiles. KCN is predominantly ionic (K⊕C ≡ N) and provides cyanide ions. Both carbon and nitrogen are capable of donating electron pair. C-C Bond being stronger than C-N bond, attack occurs through carbon atom of cyanide group forming alkyl cyanides as major product. However AgCN (Ag-C ≡ N) is mainly covalent compound and nitrogen is free to donate pair of electron. Hence attack occurs through nitrogen resulting in formation of isocyanide. Another ambident nucleophile is nitrite ion, which can attack through ‘O’ or ‘N’.
|
Mechanism of SN reaction :
Leaving group : Leaving group is the group which leaves the carbon by taking away the bond pair of electrons.
Mechanism of SN reaction :
- The substrate undergoes two changes during a SN
- The original C-X bond undergoes heterolysis and a new bond is formed between the carbon and the nucleophile using two electrons of the nucleophile. These changes may occur in one or more steps.
- The description regarding the sequence and the way in which these two changes take place in SN reaction is called mechanism of SN
- Two mechanisms are observed in various SN reactions. These are denoted as SN1 and SN2
Nucleophilic bimolecular reaction (SN2) : The substitution reaction in which a nucleophile reacts with the substrate and the rate of the reaction depends on the concentration of the substrate and the nucleophile is called a nucleophilic bimolecular reaction.
SN2 mechanism of methyl bromide using aqueous KOH. OR The mechanism of alkaline hydrolysis of methyl bromide or Bromomethane. :
Consider alkaline hydrolysis of methyl bromide (Bromomethane ), CH3Br with aqueous NaOH or KOH.
CH3—Br + OH— → CH3—OH + Br
Stereochemistry and Kinetics of the reaction (R.D.S.) : This hydrolysis reaction takes place only in one step which is a rate determining step i.e. R.D.S. The rate of hydrolysis reaction depends on the concentration of CH3Br and OH— which are present in the R.D.S. of the reaction.
Rate = R = k[CH3Br] [OH—], where k is rate constant of the reaction.
SN2 reaction : The reaction between methyl bromide and hydroxide ion to form methanol follows a second order kinetics, since the rate of the reaction depends on the concentrations of two reacting species, namely methyl bromide and hydroxide ion it is bimolecular second order (2nd) Nucleophilic Substitution reaction denoted by SN2.
Salient features of SN2 mechanism :
(i) It is a single step mechanism. The reaction takes place in the following steps :
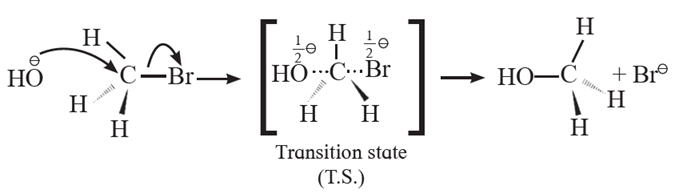
Fig. : Backside attack of nucleophile in SN2 mechanism
(ii) Backside attack of the nucleophile : Nucleophile, OH— attacks carbon atom of CH3Br from back side i.e. from opposite side to that of the leaving group i.e. Br— to experience minimum steric repulsion and electrostatic repulsion between the incoming nucleophile (OH—) and leaving Br—.
(iii) Transition state : When a nucleophile, OH— approaches carbon atom of CH3Br, the potential energy of the system increases until a transition state (T.S.) of maximum potential energy is formed in which C—Br bond is partially broken and C—OH bond is partially formed. The negative charge is equally shared by both incoming nucleophile —OH— and outgoing, leaving group -Br—. (Thus, the total negative charge is diffused.)
(iv) In CH3Br, carbon atom is sp3-hybridized and CH3Br molecule is tetrahedral. The hybridisation of carbon atom changes to sp2-hybridisation. The transition state contains carbon having three a (sigma) bonds in one plane making bond angles of 120° with each other i.e., H1, H2 and H3 atoms lie in one plane while two partial
covalent bonds containing Br and OH lie collinear and on opposite sides perpendicular to the plane.
(v) Inversion of configuration :
- The transition state decomposes fast by the complete breaking of the C—Br bond and the new C—OH bond is formed on the other side.
- The breaking of C—Br bond and the formation of C—OH bond take place simultaneously.
- The energy required to break the C—Br bond is partly obtained from the energy released when C—OH bond is formed.
- The formation of product CH3OH is accompanied by complete or 100% inversion of configuration forming again sp3-hybridized carbon atom giving tetrahedral CH3OH molecule. But in this structure the positions of H2 and H3 atoms in the reactant (CH3Br) and in product are on the opposite side.
- This inversion of configuration is called Walden inversion.
SN1 reaction : The substitution reaction in which a nucleophile reacts with the substrate and the rate of the reaction depends only on the concentration of the substrate is called nucleophilic unimolecular or first order reaction or SN1 reaction.
- The reaction between tert-butyl bromide and hydroxide ion to give tert-butyl alcohol follows a first-order kinetics, that is the rate of this reaction depends on concentration of only one species, which is the substrate molecule, tert-butyl bromide. Hence it is called substitution nucleophilic unimolecular, SN1.
Consider alkaline hydrolysis of tert-butyl bromide (2-Bromo-2-methylpropane) with aqueous NaOH or KOH

Kinetics of the reaction : Due to steric hindrance of voluminous three methyl groups around carbon, nucleophile OH— cannot attack carbon atom directly. Hence, the reaction takes place in two steps.
Step I : This involves heterolytic fission of C—Br covalent bond in the substrate forming planar carbocation and Br—. This is a slow and reversible process.

Step II : This step involves attack of nucleophile OH— or carbocation fonning C—OH bond and product tert-butyl alcohol. Since it involves ionic charge neutralisation, it is a fast step.
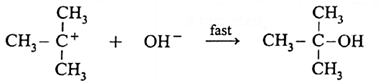
Rate Determining Step (R.D.S.) : Since the first step is a slow step, it is R.D.S., and therefore the rate of the reaction depends on the concentration of only one reactant, (CH3)3C—Br.
Rate = R = k [(CH3)3C—Br] where k is a rate constant of the reaction.
Stereochemistry and mechanism of the reaction : The reaction takes place in two steps and both the steps involve formation of transition states (T.S.).
T.S.-I for first step :
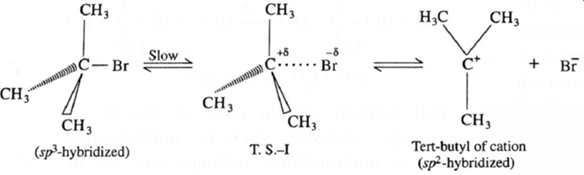
- In this transition state, C—Br bond is partially broken, so that carbon atom carries partial positive Charge (+ δ) and Br carries partial negative charge ( — δ) which further breaks forming carbocation and Br—
- Tart-butyl cation (carbocation) has a planar structure and the CH3 —C—CH3 bond angle is 120°.
- It is the intermediate of the reaction. It is unstable. In this step, hybridisation of carbon atom changes from sp3 (tetrahedral geometry) to sp3 (planar geometry).
T.S.—II for second step :
In this transition state, C—OH bond is partially formed so that carbon atom carries partial positive charge (+δ) and OH carries partial negative charge (—δ) which further forms tert-butyl alcohol.
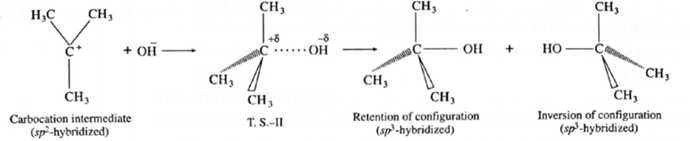
Fig. SN1 mechanism
Formation of a racemic mixture : Since OH— has equal probability of the attack on carbocation from front side and from backside, the products obtained are equal. In case of optical active alkyl halide, a racemic mixture is obtained.
Salient features of SN1 mechanism :
- Two step
- Heterolyis of C-X bond in the slow and reversible first step to form planar
- carbocation intermediate.
- Attack of the nucleophile on the carbocation intermediate in the fast second step to form the product.
- When SN1 reaction is carried out at chiral carbon in an optically active substrate, the product formed is nearly racemic. This indicates that SN1 reaction proceeds mainly with racemization. This means both the enantiomers of product are formed in almost equal amount. Racemization in SN1 reaction is the result of formation of planar carbocation intermediate. Nucleophile can attack planar carbocation from either side which results in formation of both the enantiomers of the product.
Factors influencing SN1 and SN2 mechanism :
(i) Nature of substrate :
- SN2 : The T.S. of SN2 mechanism is penta-coordinate and thus crowded. As a result SN2 mechanism is favoured in primary halides and least favoured in tertiary halides.
- SN1 : A planar carbocation intermediate is formed in SN1 reaction. It has no steric crowding. Bulky alkyl groups can be easily accommodated in planar carbocation. As a result SN1 mechanism is most favoured in tertiary halides and least favoured in primary halides.
- The carbocation intermediate is stabilized by + effect ofalkyl substituents and also by hyperconjugation effect of alkyl substituents containing α-hydrogens. As a result, SN1 mechanism is favoured in tertiary halides and least favoured in primary halides.
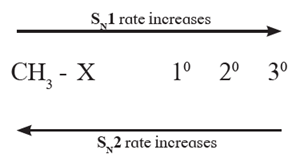
- Thus, tertiary alkyl halides undergo nucleophilic substitution by SN1 mechanism while primary halides follow SN2
(ii) Nucleophilicity of the reagent :
- A strong nucleophile attacks the substrate faster and favours SN2 The rate of SN1 mechanism is independent of the nature of nucleophile. Nucleophile does not react in the 1st step (slow step) of SN1. Nucleophile reacts fast after the carbocation intermediate is formed.
(ii) Solvent polarity :
- SN1 reaction proceeds more rapidly in polar protic solvents than in aprotic solvent. Polar protic solvent decreases the rate of SN2
- In SN2 mechanism, rate depends on substrate as well as nucleophile. A polar solvent stabilizes nucleophile by solvation. Thus solvent deactivates the nucleophile by stabilizing it. Hence, aprotic solvents or solvent of low polarity will favour SN2 mechanism.
Know This :
|
Question. Primary allylic and primary benzylic halides show higher reactivity by SN1 mechanism than other primary alkyl halides. Explain.
SN1 reaction involves formation of carbocation intermediate. The allylic and benzylic carbocation intermediate formed are resonance stabilized, and hence SN1 mechanism is favoured.

Question. Which of the following two compounds would react faster by SN2mechanism and Why ?
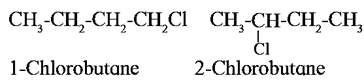
In SN2 mechanism, a pentacoordinate T.S. is involved. The order of reactivity of alkyl halides towards SN2 mechanism is, Primary > Secondary > Tertiary, (due to increasing crowding in T.S. from primary to tertiary halides. 1-Chlorobutane being
primary halide will react faster by SN2 mechanism, than the secondary halide 2-chlorobutane.
Elimination reaction : Dehydrohalogenation
When alkyl halide having at least one β-hydrogen is boiled with alcoholic solution of potassium hydroxide, it undergoes elimination of hydrogen atom from β-carbon and halogen atom from α-carbon resulting in the formation of an alkene. This reaction is called β-elimination (or 1,2 - elimination) reaction as it involves elimination of halogen and a β – hydrogen atom.
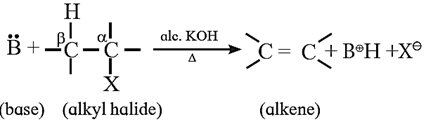
Example:
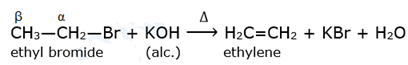
Tertiary butyl bromide when heated with alcoholic solution of potassium hydroxide forms isobutylene
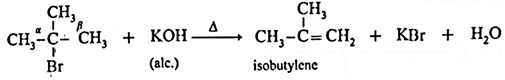
This reaction involves elimination of halogen and a β-hydrogen atom. As hydrogen and halogen is removed in this reaction it is also known as dehydrohalogenation reaction.
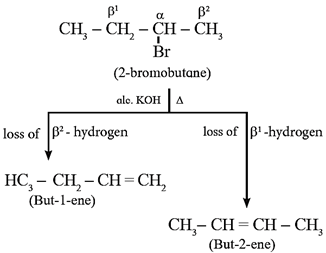
Action of alc. KOH on 2-bromobutane :
If there are two or more non-equivalent β-hydrogen atoms in a halide, then this reaction gives a mixture of products. Thus, 2-bromobutane on heating with alcoholic KOH gives mixture of but-1-ene and but-2-ene.
Saytzeff’s rule :
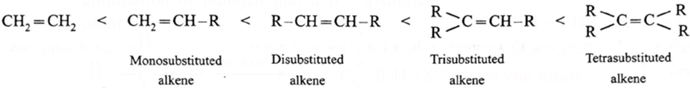
- In dehydrohalogenation reaction the preferred product is that alkene which has the greater number of alkyl groups attached to the doubly bonded carbon atoms.
- Hence the number of alkyl substituents on doubly bonded carbon atoms increases, the stability of the alkene giving its major products.
Hence the increasing stability of alkenes is,
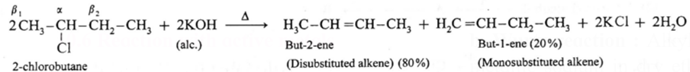
There are two types of β hydrogens (β1 and β2) therefore two alkenes are expected
| Know This :
Elimination versus substitution: Alkyl halides undergo sunstitution as well as elimination reaction. Both reactions are brought about by basic reagent, hence there is always a competition between these two reactions. The reaction which actually predominates depends upon following factors.
|
Question. Convert Ethanol to propane nitrile.
CH3—CH2—OH + HBr (48%) → CH3—CH2—Br + H2O
CH3—CH2—Br + KCN \(\underrightarrow{alc.}\) CH3—CH2—CN + KBr
Question. Convert But-1-ene to n-butyl iodide.
6CH3—CH2—CH=CH2 + B2H6 → 2(CH3—CH2—CH2—CH2)3B
(CH3—CH2—CH2—CH2)3B + 3H2O2 \(\frac{OH^-}{H_2O}\)> 3CH3—CH2—CH2—CH2—OH + B(OH)3
3CH3—CH2—CH2—CH2—OH + PI3 \(\frac{red\,P/I_2}{Δ}\)> 3CH3—CH2—CH2—CH2I + H3PO3
Reaction with active metals :
Organometallic compounds : Active metals like sodium, magnesium cadmium readily combine with alkyl chlorides, bromides and iodides to form compounds containing carbon-metal bonds. These are known as organometallic compounds.
Grignard reagent : An organometallic compound in which the divalent magnesium is directly linked to an alkyl group (R—) and a halogen atom (X), and has general formula R—Mg—X is called Grignard reagent. OR
When alkyl halide is treated with magnesium in dry ether as solvent, it gives alkyl magnesium halide. It is known as Grignard reagent.
R—X + Mg \(\underrightarrow{dry\,,ether}\) R—Mg—X
- The carbon-magnesium bond is highly polar and magnesium-halogen bond is in ionic in nature.
- Grignard reagent is highly reactive.
- It is an important reagent and used in the preparation of a large number of organic compounds.
Preparation of Grignard reagent :
(i) When an alkyl halide like CH3—I is added from a dropping funnel to a flask containing pieces of pure Mg in pure and dry ether (dielhyl ether) and a trace of iodine, Grignard reagent, CH3—Mg—I is formed.
CH3—I + Mg \(\underrightarrow{dry\,,ether}\) CH3—Mg—I
(ii) Ethyl iodide when treated with magnesium in presence of dry ether forms ethyl magnesium iodide
C2H5—I + Mg \(\underrightarrow{dry\,,ether}\) C2H5—Mg—I
Grignard reagents’ reaction with water or compounds containing hydrogen attached to electronegative element :

Examples :
Action of water on :
(1) Methyl magnesium iodide : When methyl magnesium iodide is treated with water, methane is obtained
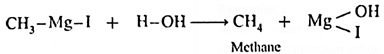
(2) Ethyl magnesium iodide : When ethyl magnesium iodide is treated with water, ethane is obtained.

| Know This :
Carbon-magnesium bond in Grignard reagent is a polar covalent bond. The carbon pulls electrons from the electropositive magnesium. Hence carbon in Grignard reagent has negative polarity and acts as a nucleophite
Victor Grignard received Nobel Prize in 1912 for synthesis and study of organomagnesium compounds. Grignard reagent is a very versatile reagent used by organic chemist. Vinyl and aryl halides also form Grignard reagent. |
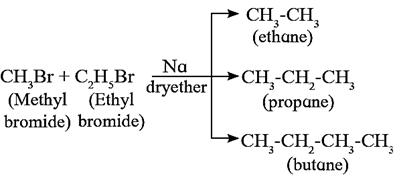
Wurtz reaction : Alkyl halides react with metallic sodium in dry ether as solvent, and form higher alkanes containing double the number of carbon atoms present in alkyl halide. This reaction is called Wurtz reaction.
2R—X + 2Na R—R + 2NaX
- In this reaction the alkyl radicals from two molecules of the reacting alkyl halide combine or couple to form the higher alkane.
Thus, methyl bromide reacts with sodium in ether to form ethane (C2H6), whlle ethyl bromide under the same conditions forms n-butane (C4H10)
2CH3Br + 2Na \(\underrightarrow{dry\,,ether}\) CH3-CH3 + 2NaBr
2C2H5Br + 2Na \(\underrightarrow{dry\,,ether}\) CH3-CH2-CH2-CH3 + 2NaBr
When a mixture of two different alkyl halides is used, all the three possible alkanes are formed.
Example: a mixture of CH3Br and C2H5Br gives propane along with C2H6 and C4H10
Reaction of haloarenes :
Wurtz- Fittig reaction : The reaction of aryl halide with alkyl halide and sodium metal in dry ether to give substituted aromatic compounds is known as Wurtz- Fittig reaction.
- This reaction is an extension of Wurtz reaction and was carried out by Fittig.
- This reaction allows alkylation of aryl halides.
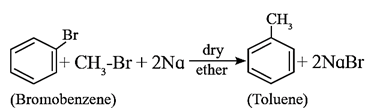
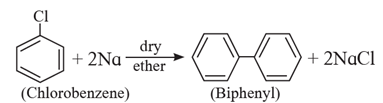
Action of aryl halide on sodium metal : In this case only aryl halide takes part in the reaction, the product is biphenyl and the reaction is known as Fittig reaction.
Nucleophilic substitution SN of haloarenes :
Haloarenes (Aryl halides) show low reactivity towards nucleophilic substitution reactions. The low reactivity of aryl halides is due to :
- Resonance effect and
- sp2 hybrid state of C .
Resonance effect : In haloarenes, the electron pairs on halogen atom are in conjugation with π-electrons of the benzene ring. The delocalization of these electrons C-Cl bond acquires partial double bond character.
For example, different resonance structures of chlorobenzene.

Resonance structures II, III and IV show double bond character to carbon-chlorine bond.
- Due to partial double bond character of C-Cl bond in aryl halides, the bond cleavage in haloarene is difficult and are less reactive. On the other hand, in alkyl halides, carbon is attached to chlorine by a single bond and it can be easily broken.
- Aryl halides are stabilized by resonance but alkyl halides are not. Hence, the energy of activation for the displacement of halogen from aryl halides is much greater than that of alkyl halides.
sp2 hybrid state of C :
Different hybridization state of carbon atom in C-X bond :
- In alkyl halides. the carbon of C-X bond is sp3-hybridized with less s-character and greater bond length of 178 pm. which requires less energy to break the C-X bond.
- In aryl halides the carbon of C-X bond is sp2-hybridized with more s-character and shorter bond length which requires more energy to break C-X bond. Therefore. aryl halides are less reactive than alkyl halides.
- Polarity of the C-X bond : In aryl halide C-X bond is less polar than in alkyl halides. Because sp2-hybrid carbon of C-X bond has less tendency to release electrons to the halogen than a sp3-hybrid carbon in alkyl halides. Thus halogen atom in aryl halides cannot be easily displaced by nucleophile.
Instability of Phenyl cation :
- Phenyl cation produced due to selfionization of haloarene will not be stabilized by resonance, which rules out possibility of SN1
- Back side attack of nucleophile is blocked by the aromatic ring, which rules out SN2
- Thus cations are not formed and hence aryl halides do not undergo nucleophilic substitution reaction easily.
π-bond :
- As any halides are electron rich molecules due to the presence of π-bond, they repel electron rich nucleophilic attack. Hence, aryl halides are less reactive towards nucleophilic substitution reactions.
- However, the presence of electron withdrawing groups at o/p position activates the halogen of aryl halides towards substitution.
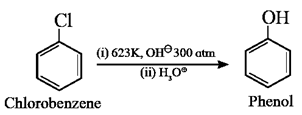
Electrophilic substitution (SE) in aryl halides :
Aryl halides undergo electrophilic substitution reactions slowly and it can be explained as follows :
Inductive effect : The strongly electronegative halogen atom withdraws the electrons from carbon, atom of the ring (—I effect). This deactivates the ring hence aryl halides show reactivity towards electrophilic attack.
Resonance effect : The resonating structures of aryl halides show increase in electron density at ortho and para position, hence it is o, p directing.

The inductive effect and resonance effect compete with each other. The inductive effect is stronger than resonance effect. The reactivity of aryl halides is controlled by stronger inductive effect and o, p orientation is controlled by weaker resonating effect.
The attack of electrophile (Y) on haloarenes at ortho and para positions are more stable due to formation of chloronium ion. The chloronium ion formed is comparatively more stable than other hybrid structures of carbonium.
Substitution reaction of chlorobenzene :
- Aryl halides undergo electrophilic substitution reaction slowly as compared to
- In resonance structures of chlorobenzene elelctron density is relatively more at ortho and para position. Therefore incoming electrophilic group is more likely to attack at these positions. But due to steric hinderance at ortho position, para product usually predominates.
- In haloarenes, halogen atom has strong electron withdrawing inductive effect (-I). This deactivates the ring and electrophilic substitution reaction occurs slowly.
(i) Halogenation : It is carried out by reacting haloarene with halogen in presence of ferric salt as Lewis acid catalyst.
(ii) Nitration : It is carried out by heating haloarene with conc. HNO3 in presence of conc. H2SO4.
(iii) Sulfonation : It is carried out by heating haloarene with fuming H2SO4.
(iv) Friedel Craft’s reaction : It is carried out by treating haloarene with alkyl chloride or acyl chloride in presence of anhydrous AlCl3 as a catalyst.
- Methyl chloride in the presence of anhydrous AlCl3 : When chlorobenzene is treated with methyl chloride in the presence of anhydrous AlCl3, a mixture of 1-chloro-4-methyl benzene (major product) and 1-chloro-2-methyl benzene is formed. Since, the alkyl group is introduced in the benzene ring, the reaction is termed as Friedel Craft’s alkylation.
- Acetyl chloride in the presence of anhydrous AlCl3 : When chlorobenzene is reacted with acetyl chloride in the presence of anhydrous AlCl3, a mixture of 2-chloro acetophenone and 4-chloro acetophenone (major product) is Since, the acetyl group is introduced in the benzene ring, the reaction is termed as Friedel Craft’s acylation.
Uses and Environmental effects of some polyhalogen compounds :
(i) Dichloromethane/ methylene chloride (CH2Cl2) :
It is a colourless volatile liquid with moderately sweet aroma.
Uses :
- Dichloromethane dissolves wide range of organic compounds, hence it is used as solvent for many chemical reactions.
- It is used as a solvent as a paint remover and degreaser.
- It is used as propellant in aerosols and as a fumigant pesticide for grains and strawberries.
- It is used to decaffinate tea or coffee.
Environmental effects :
- Higher levels of dicliloromethane in air causes nausea, numbness in fingers and toes, dizziness
- Lower levels of dichloromethane causes impaired vision and hearing.
- Direct contact with eyes can damage cornea.
(ii) Chloroform / trichloromethane (CHCl3) :
It is a colourless liquid with peculiar sweet smell.
Uses :
- Chloroform in the production of chlorofluoromethane, freon refrigerant R-22.
- It is used as solvent in pharmaceuticals, pesticides, gums, fats, resins and dye industry.
- It is a good source of dichlorocarbene species.
Environmental effects :
- When chloroform is exposed to air in the presence of sunlight, it slowly oxidised to phosgene, a poisonous compound, therefore it is stored in dark, amber coloured bottles.
- Chloroform vapour when inhaled for a short time causes dizziness, headache and fatigue and if inhaled for a long time affects central nervous system.
(iii) Tetrachloromethane or carbon tetrachloride (CCl4) :
It is a colourless liquid with sweet smell.
Uses :
- Carbon tetrachloride is used in the manufacture of refrigerants.
- It is used as a dry cleaning agent and as a pesticide for stored grains.
- It is very useful solvent for oils, fats and resins. It senses as a source of chlorine.
Environmental effects :
- Exposure to carbon tetrachloride causes eye irritation, damages nerve cells, vomiting sensation, dizziness, unconciousness or death. Long exposure to chloroform may affect liver.
- When mixed with air it causes depletion of the ozone layer, which affects human skin leading to cancer.
(iv) Iodoform or triiodomethane (CHI3) :
It is a yellow crystalline substance with disagreeable smell.
Uses :
- Iodoform is used as antiseptic, dressing of wounds and sores.
- On small scale it is used as disinfectant.
Environmental effects :
- Iodoform has a strong smell. It causes irritation to skin and eyes. It may cause respiratory irritation or breathing difficulty, dizziness, nausea, depression of central nervous system, visual disturbance.
(v) Freons :
These are organic compounds of chlorine and fluorine, chlorofluorocarbons,
The most common representative is dichlorodifluromethane (Freon-12) others
include chlorodifluromethane (R-22), trichlorofluromethane (R-11)
Uses :
- Freons are widely used as propellants in aerosol, products of food, cosmetics and pharmaceutical industries.
- Freons containing bromine in their molecules are used as fire extinguishers.
- They are used in aerosol insecticides, solvent for cleaning clothes and metallic surfaces. It is used as foaming agents in the preparation of foamed plastics and in production of certain fluorocarbons.
- It is used as refrigerants and air conditioning purposes.
Environmental effects :
- Freon as refrigerant causes ozone depletion.
- Freons have low toxicity and low biological activity.
- Freons from propane group are more toxic in nature.
- Regular large inhalation of freon results in breathing problems, organ damage, loss of consciousness.
(vi) Dichlorodiphenyltrichloroethane (DDT) :
It is colourless, tasteless and odorless crystalline compound having insecticidal property.
Uses :
- DDT is used as insecticide against malaria and typhus.
- It is used to kill various insects like housefly and mosquitoes
Environmental effects :
- DDT is not readily metabolised by animals.
- It is deposited and stored in fatty tissues.
- Exposure to high doses of DDT may cause vomiting, tremors or shakiness.
- Laboratory animal studies showed adverse effect of DDT on liver and reproduction.
- DDT is a pressistent organic pollutant, readily absorbed in soils and tends to accumulate in the ecosystem.
- When dissolved in oil or other lipid, it is readily absorbed by the skin. It is resistant to metabolism. There is a ban on use of DDT due to all these adverse effects.
| Know This :
Q. How do CFC distroy the ozone layer in the atmosphere ?
Answer :
When ultraviolet radiation (UV) strikes CFC (CFCl3) molecules in the upper atmosphere, the carbon-chlorine bond breaks and produces highly reactive chlorine atom (Cl). CFCl3 → CFCl2 + Cl This reactive chlorine atom decomposes ozone (O3) molecule into oxygen molecule (O2). O3 + Cl → O2 + ClO ClO + O → O2 + Cl One atom of chlorine can destroy upto 100,000 ozone molecules.
|
PDF : Chapter-10-Halogen Derivatives-Text Book
PDF : Chapter-10-Halogen Derivatives- Notes
PDF : Chapter-10-Halogen Derivatives- Solution
All 16 Chapters Notes -Class-12-Chemistry (16-PDF)
All 16 Chapters Solutions -Class-12-Chemistry (16-PDF)
All 16 Chapters Notes+Solutions -Class-12-Chemistry (32-PDF)
Main Page : – Maharashtra Board Class 12th-Chemistry – All chapters notes, solutions, videos, test, pdf.
Previous Chapter : Chapter 9- Coordination Compounds – Online Notes
Next Chapter : Chapter-11-Alcohols, Phenols and Ethers- Online Notes
We reply to valid query.