Halogen Derivatives
Maharashtra Board-Class-12-Chemistry-Chapter-10
Notes-Part-1
Topics to be Learn : Part-1
|
Halogen derivatives : Replacement of hydrogen atom/s in aliphatic or aromatic hydrocarbons by halogen atom/s results in the formation of halogen derivatives of hydrocarbons.
Classification of halogen derivatives
Halogen derivatives of hydrocarbons are classified mainly in two ways.
- On the basis of hydrocarbon skeleton to which halogen atom is bonded.
- On the basis of number of halogen atoms.
Halogen derivatives of alkane are classified as
(i) Monohalogen derivatives :
- (Alkyl halides)
- (Allylic halides)
- (Benzylic halides)
- (Vinylic halides)
- (Haloalkyne)
- (Aryl halides)
(ii) Polyhalogen derivatives
- Dihalogen derivatives : (a) Vicinal dihalides (b) Geminal dihalides
- Trihalogen derivatives
- Tetrahalogen derivatives
(1) Monohalogen derivatives : Monohalogen derivative (or alkyl halide) : It is a halogen derivative of an alkane in which one hydrogen atom is replaced by one halogen atom and it is also called alkyl halide. E.g. C2H5Br
(2) Polyhalogen derivatives : These are halogen derivatives in which more than one hydrogen atoms of an alkane are substituted by corresponding number of halogen atoms. They are classified as follows :
(i) Dihalogen derivatives : The compounds formed by the substitution of two hydrogen atoms of an alkane by two halogen atoms are called dihalogen derivatives.

Example : CH2CL2
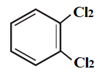
They are further classified as :
- Vicinal dihalides : (Two halogen atoms on vicinal or adjacent carbon atoms)
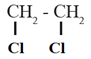
- Geminal dihalides : CH3—CHCl2 (Two halogen atoms on the same carbon atom)
(ii) Trihalogen derivatives : The compounds formed by the substitution of three hydrogen atoms of an alkane by three halogen atoms are called trihalogen derivatives.
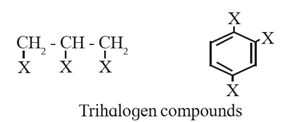
Example : CHCL3
(iii) Tetrahalogen derivatives : The compounds formed by the substitution of four hydrogen atoms of an alkane by four halogen atoms are called tetrahalogen derivatives. E. g. CCl4.
Classification of monohalogen compounds :
Monohalogen compounds are further classified on the basis of position of halogen atom and the type of hybridization of carbon to which halogen is attached.
(i) Alkyl halides or haloalkanes : The compound formed by the replacement of one hydrogen atom in an alkane by a halogen atom is called an alkyl halide.
In alkyl halides or haloalkanes the halogen atom is bonded to sp3 hybridized carbon
Alkyl halides are classified into the following three classes depending on the type of the carbon to which halogen atom is bonded.
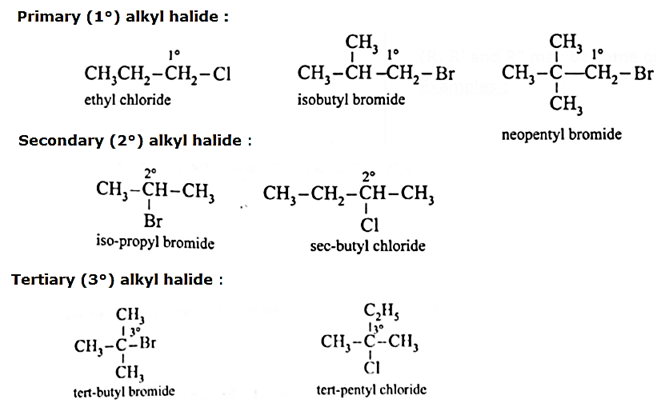
- Primary (1°) alkyl halide : Alkyl halide in which a halogen atom is bonded to a primary carbon atom is called primary alkyl halide. [Primary (1°) carbon atom i.e., the carbon atom which is attached to only one carbon atom.]
- Secondary (2°) alkyl halide : Alkyl halide in which a halogen atom is bonded to a secondary carbon atom is called secondary alkyl halide. [Secondary (2°) carbon i.e., the carbon atom which is attached to two other carbon atoms.]
- Tertiary (3°) alkyl halide : Alkyl halide in which halogen atom is bonded to a tertiary carbon atom is called tertiary alkyl halide. [Tertiary (3°) carbon i.e., the carbon atom which is attached to three other carbon atoms.]
General formula for Primary, Secondary and Tertiary halide :
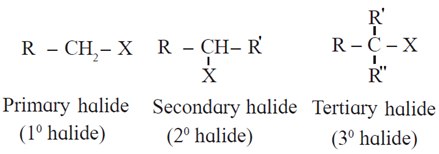
(R, R’ and R" may be same or different)
Examples :
(ii) Allylic halides : In allylic halides, halogen atom is bonded to a sp3 hybridized carbon atom next to a carbon-carbon double bond.
Example : CH2=CH—CH2-Br (Allyl bromide)
(iii) Benzylic halide : In benzylic halides halogen atom is bonded to a sp3 hybridized carbon atom which is further bonded to an aromatic ring.
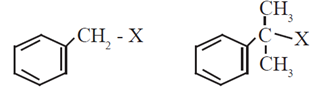
(iv) Vinylic halides : In vinylic halides halogen atom is bonded to a sp2 hybridized carbon atom of aliphatic chain. Vinylic halide is a haloalkene.
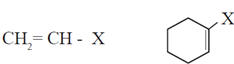
(v) Haloalkyne : When a halogen atom is bonded to a sp hybridized carbon atom it is a haloalkyne.
CH ≡ C - X
(vi) Aryl halides or haloarenes : In aryl halides, halogen atom is directly bonded to the sp2 hybridized carbon atom of aromatic ring.
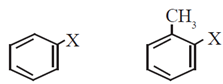
Nomenclature of halogen derivatives
- According to IUPAC system of nomenclature alkyl halides are named as haloalkanes. Aryl halides are named as haloarenes in common as well as IUPAC system.
- For dihalogen derivative of an arene, prefix o-, m-, p- are used in common name system but in IUPAC system the numerals 1,2 ; 1,3 and 1,4 respectively are used.
Common and IUPAC names of some halogen derivatives :
| Formula | Common name | IUPAC name |
| CH2Cl2 | Methylene chloride | Dichloromethane |
| CH3CH2Br | Ethyl bromide | Bromoethane |
| CH3CH(Cl)CH3 | Isopropyl chloride | 2-Chloropropane |
| (CH3)2 CH - CH2Br | Isobutyl bromide | 1-Bromo-2-methylpropane |
| (CH3) 3 C Br | Tert-butyl bromide | 2-Bromo-2-methyl-propane |
| (CH3) 3 C CH2Cl | Neopentyl chloride | 1-Chloro-2, 2-dimethyl pro-
pane |
| CH2 = CH - Cl | Vinyl chloride | 1-Chloroethene |
| CH2 = CH - CH2Br | Allyl bromide | 3-Bromopropene |
| CH ≡ C - Cl | Chloro acetylene | Chloroethyne |
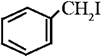 |
Benzyl iodide | Iodophenylmethane |
 |
p-Iodotoluene | 1-Iodo-4-methyl benzene or 4-Iodotoluene |
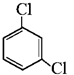 |
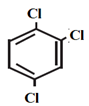
m-dichlorobenzene | 1, 3-dichlorobenzene |
Examples of compound and structure :

Possible isomers of monochlorodcrivatives of 2,3-Dimethylbutane and their IUPAC names :
Molecular formula : C6H14
The monochloroderivative of this compound has molecular formula C6H13Cl
The parent hydrocarbon is,

Methods of preparation of alkyl halides :
(1) From alcohol : The most widely used method of preparation of alkyl halide is replacement of hydroxyl group of an alcohol by halogen atom.
- Alcohols are available in a wide variety. The hydroxyl group may be replaced by halogen atom using, (a) halogen acid, (b) phosphorous halide or (c) thionyl chloride.
Compounds obtained from alcohols :
By using halogen acid or hydrogen halide (HX) :
- Alcohols in the presence of Lucas reagent which is a solution of concentrated HCl and ZnCl2 form alkyl halides.
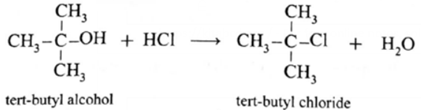
- Hydrogen chloride is used with zinc chloride (Grooves' process) for primary and secondary alcohols, but tertiary alcohols readily react with concentrated hydrochloric acid in absence of zinc chloride.
- The order of reactivity of alcohols with a given haloacid is 30>20>10.
Examples :
(i) ethyl chloride C2H5Cl
C2H5—OH (ethyl alcohol) + HCI (conc.) \(\frac{anyhydrus\,,ZnCl_2}{Δ}\)> C2H5—Cl (chloroethane) + H2
(ii) isopropyl chloride (CH3—CHCl—CH3)

(iii) tert-butyl chloride (CH3)3—Cl
- Constant boiling hydrobromic acid (48%) is used for preparing alkyl bromides.
- Primary alkyl bromides can also be prepared by reaction with NaBr and H2SO4. Here HBr is generated in situ.
Examples :
(1) ethyl bromide (C2H5Br)
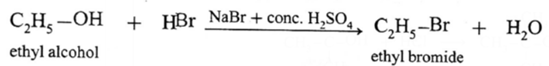
(2) isopropyl bromide (CH3 — CHBr — CH3)
Isopropyl alcohol, on reaction with NaBr and dil. H2SO4 forms isopropyl bromide
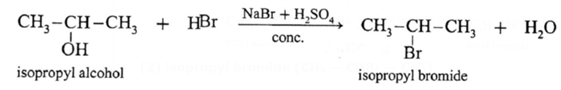
(3) tert-butyl bromide (CH3)3C—Br
Tertiary alcohol on reaction with sodium bromide and dil. H2SO4 forms ten-butyl bromide
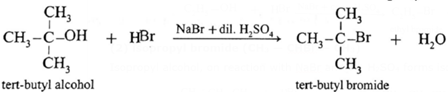
By using phosphorous halide :
- An alkyl halide may be prepared by action of phosphorous halide on alcohol.
- Phosphorous tribromide and triiodide are usually generated in situ (produced in the reaction mixture) by the action of red phosphorous on bromine and iodine respectively.
- Phosphorous pentachloride reacts with alcohol to give alkyl chloride.
Examples :
(1) ethyl iodide obtained from ethyl alcohol :
When ethyl alcohol is treated with sodium or potassium iodide in 95% phosphoric acid, ethyl iodide is formed. Here HI is generated in situ.
CH3-CH2-OH + HI \(\frac{NaI/(H_3PO_4)}{Δ}\)> CH3—CH2—I + H2O
Know This :
|
By using thionyl chloride :
- Thionyl chloride reacts with straight chain primary alcohols to give un-rearranged alkyl chloride.
- The byproducts obtained are gases. There is no need to put extra efforts for its separation. Therefore this method is preferred for preparation of alkyl chloride.
Example :
Preparation of Ethyl chloride (chloroethane) from ethyl alcohol using (i) PCl3 (ii) PCl5 and (iii) SOCl2
(i) When ethyl alcohol is refluxed with phosphorus trichloride, ethyl chloride is formed.
3CH3CH2OH + PCl3 \(\underrightarrow{Δ}\) 3CH3CH2Cl + H3PO3
(ii) When ethyl alcohol is refluxed with phosphorus pentachloride, ethyl chloride is formed.
CH3CH2OH + PCl5 \(\underrightarrow{Δ}\) CH3CH2Cl + POCl3 + HCl
(iii) When ethyl alcohol is refluxed with thionyl chloride, in the presence of pyridine, ethyl chloride is formed. The by-products obtained are gases. Therefore, this method is preferred for preparation of alkyl chloride.
CH3CH2OH + SOCl2 \(\frac{pyridine}{reflux}\)> CH3CH2Cl + SO2 + HCl
(2) From hydrocarbon :
Alkyl halides are formed from saturated as well as unsaturated hydrocarbons by various reactions.
(i) Halogenation : A reaction of alkanes with halogens (Cl2, Br2, I2) 1n the presence of appropriate conditions forming a mixture of alkyl halides and poly halogen compounds.
(ii) Chlorination :
CH4 + Cl2 \(\underrightarrow{UV\,,light}\) CH3-Cl + HCl
- When excess of chlorine is used, tetrachloro methane, a major product is obtained. When excess of methane is used, chloromethane, a major product is obtained.
- The order of reactivity of halogens towards alkane is F2 > Cl2 > Br2 > I2.
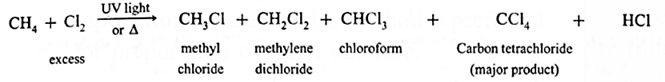
(iii) Iodination :
CH4 + I2 ⇌ CH3—I + HI
methyl iodide
- However, iodination reaction is a reversible reaction. HI being a strongest reducing agent reduces methyl iodide back to methane.
(iv) Fluorination : A reaction of alkane with fluorine is explosive and also hydrofluoric acid is poisonous and corrosive. Hence, alkyl fluorides are not prepared by halogenation of alkane.
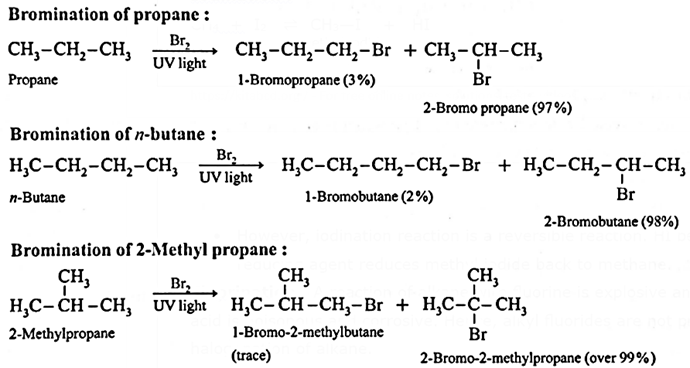
Disadvantages of Halogenation of alkanes for preparation of alkyl halides :
- Direct fluorination of alkanes is highly exothermic, explosive and invariably leads to polyfluorination and decomposition of the alkanes. It is difficult to control the reaction.
- Direct iodination of alkanes is highly reversible and difficult to carry out.
- In direct chlorination and bromination, the reaction is not selective. It can lead to different isomeric monohalogenated alkanes (alkyl halides) as well as polyhalogenated alkanes.
Hence, halogenation of alkanes is not a good method of preparation of alkyl halides.
Q. Why, direct iodination of alkanes is not possible ?
- Direct iodination of alkanes using iodine is highly reversible. RH + I2 ⇌ RI + HI
- Hydroiodic acid Hl being strong reducing agent, it reduces RI to alkane RH.
- The reaction takes place only in the presence of a suitable oxidizing agent like HgO, HIO3 or dilute HNO3 which decomposes HI.
- Hence, direct iodination of alkanes is not possible.
5C2H6 + 2I2 + HIO3 → 5C2H5—I + 3H2O
Markovnikov’s rule : When an unsymmetrical reagent is added to an unsymmetrical alkene, the negative part of the reagent gets attached to that carbon atom of the double bond which canies less number of hydrogen atoms.
Example : Addition of HBr to unsymmetrical alkene like propene gives two products.
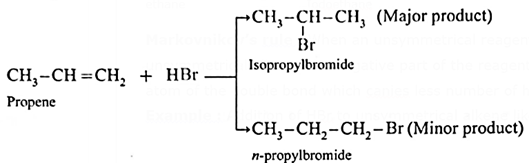
Kharasch-Mayo effect (Peroxide effect) :
The addition of HBr to an unsymmetrical alkene (propene) in the presence of benzoyl peroxide takes place in the opposite orientation to that of Markovnikov’s rule and this is known as Kharasch-Mayo effect or peroxide effect or Anti-Markovnikov addition.
H3C—CH=CH2 + H—Br \(\frac{(C_6H_5CO)_2O_2}{Benzoyl\,,peroxide}\)> H3C—CH2—CH2—Br (1-Bromopropane )
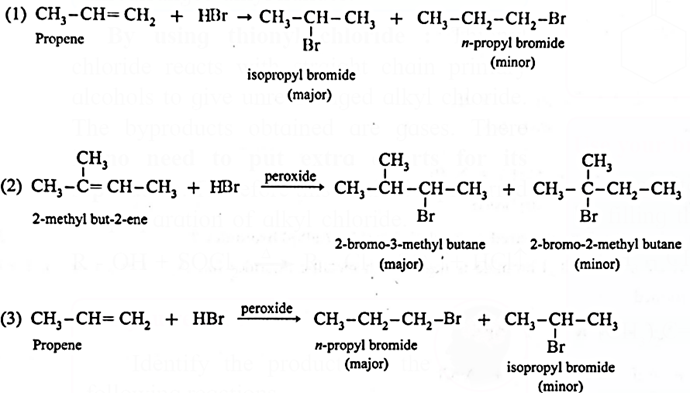
Q. Rewrite the following reaction by filling the blanks :
(1) CH3-CH=CH2 + HBr → ……. + ……
(major) (minor)
(2) (CH3)2C=CHCH3+ HBr \(\underrightarrow{peroxide}\) ……. + …..
(major) (minor)
(3) CH3-CH=CH2 + HBr \(\underrightarrow{peroxide}\) ……. + …..
(major) (minor)
| Know This :
When alkenes are heated with Br2 or Cl2 at high temperature, hydrogen atom of allylic carbon is substituted with halogen atom giving allyl halide. CH2=CH-CH3 + Cl2 → CH2=CH-CH2Cl + HCl |
Halogen exchange :
Finkelstein reaction : Alkyl iodides are prepared conveniently by treating alkyl chlorides or bromides with sodium iodide in methanol or acetone solution. The sodium bromide or sodium chloride precipitates from the solution and can be separated by filtration.
R-Cl + NaI \(\underrightarrow{acetone}\) R-I + NaCl ↓
The reaction is known as Finkelstein reaction.
Swartz reaction : Alkyl fluorides are prepared by heating alkyl chlorides or bromides with metal fluorides such as AgF, Hg2F2, AsF3, SbF3 etc.
R-Cl + AgF → R-F + AgCl ↓
The reaction is known as Swartz reaction.
Electrophilic substitution :
Preparation of haloarenes using electrophilic substitution :
- When arene is treated with chlorine or bromine in dark at ordinary temperature in the presence of lewis acid as a catalyst like Fe, FeCl3 or anhydrous AlCl3, aryl chloride or aryl bromide is formed.
- When toluene is brominated in dark at ordinary temperature in the presence of iron, a mixture of ortho and para bromo tolerene is obtained.
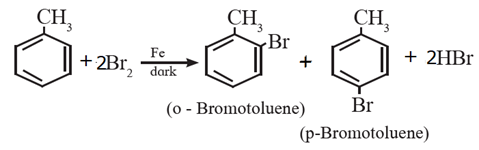
- Ortho and para isomers can be easily separated as there is large difference in melting points of ortho and para isomers.
- Aromatic electrophilic substitution with iodine is reversible. In this case use of HNO3/HIO4 removes HI by oxidation to I2, equilibrium is shifted to right and iodo product is formed.
- F2 being highly reactive, fluoro compounds are not prepared by this method.
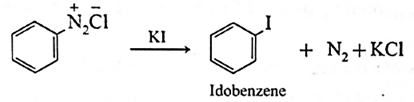
Sandmeyer’s reaction :
The replacement of diazonium group by —Cl or —Br using cuprous salt is called Sandmeyer’s reaction.
- Aryl halides are most commonly prepared by replacement of nitrogen of diazonium salt.
- When a primary aromatic amine (like aniline) suspended in cold HCl, is treated with sodium nitrite, a diazonium salt (benzene diazonium chloride) is formed.
- When diazonium salt is treated cuprous chloride or cuprous bromide, aryl halide (chlorobenzene or broinobenzene) is formed.

When benzene diazonium salt is mixed with potassium iodide, iodobenzene is formed
Physical properties :
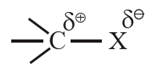
- In alkyl halide, the halogen atom is more electronegative than carbon atom, the C—X bond is polar.
(i) Nature of intermolecular forces:
- Halogens (X = F, Cl, Br and I) are more electronegative than carbon.
- Carbon atom that carries halogen develops a partial positive charge while the halogen carries a partial negative charge. Thus carbon-halogen bond in alkyl halide is a polar covalent bond. Therefore alkyl halides are moderately polar compounds.
- Size of the halogen atom increases from fluorine to iodine. Hence the C-X bond length increases.
- The C-X bond strength decreases with an increase in size of halogen. This is because as the size of p-orbital of halogen increases the p-orbital becomes more diffused and the extent of overlap with orbital of carbon decreases.
Example :
C—F bond in CH3F is the strongest bond and C-I bond in CH3I is the weakest bond.
Explanation :
- Methyl fluoride (CH3F) is highly polar molecule and has the shortest C—F bond length (139 pm) and the strongest C—F bond due to greater overlap of orbitals of the same principal quantum number i.e., overlap of 2sp3 orbital of carbon with 2pz orbital of fluorine.
- Methyl iodide (CH3I) is much less polar and has the longest (C—I) bond length (214 pm) and the weakest C—I bond due to poor overlap of 2sp3 orbital carbon with 5pz orbital of iodine i.e., 2sp3 orbital of carbon cannot penetrate into larger p-orbitals.
(ii) Boiling point :
- Boiling points of alkyl halides are considerably higher than those of corresponding alkanes due to higher polarity and higher molecular mass.
- Within alkyl halides, for a given alkyl group, the boiling point increases with increasing atomic mass of halogen, because magnitude of van der Waals force increases with increase in size and mass of halogen.
- Thus boiling point of alkyl halide decreases in the order RI > RBr > RCl > RF
For example, :
| Haloalkane | CH3F | CH3Cl | CH3Br | CH3I |
| Boiling point (K) | 194.6 | 248.8 | 276.6 | 315.4 |
- For isomeric alkyl halides (isopropyl bromide and n-propyl bromide), the boiling point decreases as the branching increases, surface area decreases on branching and van der Waals forces decrease, therefore the boiling point of isopropyl bromide is lower than that of n-propyl bromide.
- p-Diclilorobenzene has higher melting point than those of o-and m-isomers. This is because of its symmetrical structure which can easily fits in crystal lattice. As a result, intermolecular forces of attraction are stronger and therefore greater energy is required to overcome its lattice energy.
| Know This :
Bromoform, chloromethane, dibromomethane, and bromomethane these compounds contain only one carbon. Thus the molecular size depends upon the size of halogen and number of halogen atoms present. Thus increasing order of boiling point is, CH3Cl < CH3Br < CH2Br2 < CHBr3 |
(iii) Solubility :
- Though alkyl halide is polar, it is insoluble in water because alkyl halide is not able to form hydrogen bonds with water.
- Attraction between alkyl halide molecule is stronger than attraction between alkyl halide and water.
Optical isomerism in halogen derivatives :
- Isomers having the same bond connectivities, that is, structural formula are called stereoisomers.
- Optical isomerism, which is a kind of stereoisomerism is useful to understand nucleophilic substitution reactions of alkyl halides
Chiral atom and molecular chirality :
Chiral carbon atom : Carbon atom in a molecule which carries four different groups/atoms is called chiral carbon atom.
- Chiral atom in a molecule is marked with asterisk ( * ). For example, CH3-*CHCl-CH2-CH3.
Examples of Chiral carbon atoms :
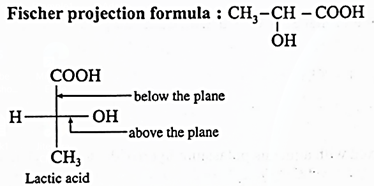
(i) Chiral carbon atoms in Lactic acid :
In lactic acid structure, the starred carbon atom is chiral carbon atom as it is attached to the four different substituents, COOH, OH, CH3 and H
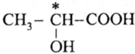
Chiral molecule : When a molecule contains one chiral atom, it acquires a unique property i.e. it is non-superimposable with its minor image is said to be chiral molecule.
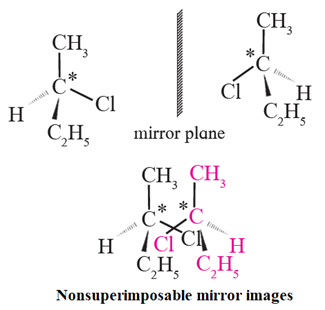
Chilarity : The relationship between a chiral molecule and its mirror image is similar to the relationship between left and right hands. Therefore it is called handedness or chirality.
Monochromatic light : It consists of rays of single wavelength vibrating in different planes perpendicular to the direction of propagation of the light.
Plane polarized light: A light having oscillations only in one plane perpendicular to direction of propagation of light is known as plane polarized light.

Optical isomerism : The steroisomerism in which the isomers have different spatial arrangements of groups/atoms around a chiral atom is called optical isomerism.
Optical activity : The property of a substance by which it rotates plane of polarization of incident plane polarized light is known as optical activity.
Optically active compound : The compound which rotate the plane of plane polarized light is called optically active compound.
Enantiomers : The optical isomers which are non-superimposable mirror images of each other are called enantiomers or enantiomoiphs or optical antipodes.
- Example : 2-chlorobutane, lactic acid
Doxtrorotatory substance or d-Isomer : An optically active substance (or isomer) which rotates the planes of a plane polarized light to the right hand side (RHS) is called dextrorotatory substance (or isomer) and denoted by d or(+) sign.
Laevorotatory substance or l-Isomer : An optically active substance (or isomer) which rotates the plane of a plane polarized light to the left hand side (LHS) is called laevorotatory substance (or isomer) and denoted by Z or (—) sign.
Racemic. mixture or Racemate : A mixture containing equimolar quantities of dextro (d) and laevo (l) optical isomers which is optically inactive due to molecules of one enantiomer is cancelled by equal and opposite optical rotation due to molecules of the other enantiomer is called a racemic mixture or racemate. It is represented as (dl) or (±)
| Remember...
· Optical activity is an experimentally observable property of compounds. Chirality is a description of molecular structure. Optical activity is the consequence of chirality. · Molecules which contain one chiral atom are chiral, that is, they are nonsuperimposable on their mirror image. · The two non-superimposable mirror image structures are called pair of enantiomers. · Enantiomers have equal and opposite optical rotation. Thus, enantiomers are a kind of optical isomers. |
A racemic mixture is optically inactive :
Explanation :
- A racemic mixture contains equimolar (or equimolecular) quantities of the dextrorotatory (d-) and laevorotatoiy (l-) isomers (enantiomers) of a compound.
- The d-enantiomer rotates the plane of plane-polarized light to the right, while the l -enantiomer rotates the same to the left to the same extent.
- The quantities of the d- and l -enantiomers being the same, both the rotations are of the same magnitude, but of opposite directions. Hence, they cancel ‘each other; Hence, a racemic mixture is optically inactive.
- It is represented as dl or( ± ). Example : ( ± ) lactic acid
Representation of configuration of molecules :
(i) Fischer projection formula (cross formula) :
- Two representations are used to represent configuration of chiral carbon and the 3-dimensional structure of optical isomers on plane paper.
- A Fischer projection formula can be drawn by visualizing the main carbon chain vertical in the molecule. Each carbon on the vertical chain is represented by a cross.
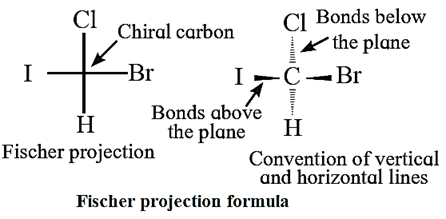
- Conventionally the horizontal lines of the cross represent bonds projecting up from the carbon and the vertical lines represent the bonds going below the carbon.
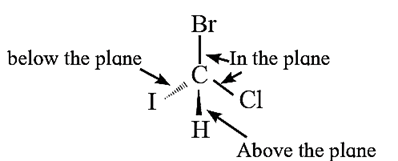
(ii) Wedge formula :
- When a tetrahedral carbon is imagined to be present in the plane of paper all the four bonds at this carbon cannot lie in the same plane.
- The bonds in the plane of paper are represented by normal lines, the bonds projecting above the plane of paper are represented by solid wedges (or simply by bold lines) while bonds going below the plane of paper are represented by broken wedges (or simply by broken lines).
Q. Draw structures of enantiomers of lactic acid using Fischer projection formulae.
Q. Draw structures of enantiomers of 2-bromobutane using wedge formula.

PDF : Chapter-10-Halogen Derivatives-Text Book
PDF : Chapter-10-Halogen Derivatives- Notes
PDF : Chapter-10-Halogen Derivatives- Solution
All 16 Chapters Notes -Class-12-Chemistry (16-PDF)
All 16 Chapters Solutions -Class-12-Chemistry (16-PDF)
All 16 Chapters Notes+Solutions -Class-12-Chemistry (32-PDF)
Main Page : – Maharashtra Board Class 12th-Chemistry – All chapters notes, solutions, videos, test, pdf.
Previous Chapter : Chapter 9- Coordination Compounds – Online Notes
Next Chapter : Chapter-11-Alcohols, Phenols and Ethers– Online Notes
We reply to valid query.